Knowde Enhanced TDS
Identification & Functionality
- Ingredient Origin
- Food Ingredients Functions
- Ingredients
- Stevia Rebaudiana Extract
- Food Additive Number
- E 960, INS 960
- Technologies
Features & Benefits
- Labeling Claims
- Food Ingredients Features
- Sugar Reduction & Glucose Management
Truvia® and ViaTech® leaf-based extracts and EverSweet™ stevia sweetener, produced sustainably via fermentation,* can reduce sugar by up to 100%, even in challenging applications.
- Functional Benefits
- Granular products available, which offer larger particle size for greater flowability & reduced dusting
- Suitable as a replacement for existing stevia formulations
- Extracted from the leaf
- Sustainably produced against Cargill Sustainability
- Standard; fully traceable
- Labeled as stevia leaf extract
Applications & Uses
- Markets
- Applications
- Food & Nutrition Applications
- Applications/Functionality
Truvia® Stevia RA95 is a high purity sweetener from the stevia leaf1 with a clean sweet taste that can be used in a wide variety of applications including beverages, fruit juices, jellies, jams, confectionary, dairy, frozen novelties, snacks, cereals, bakery chewing gum, and tabletop sweeteners. As a high intensity sweetener, Truvia® Stevia RA95 is useful in formulating products with reduced calorie and sugar content.
Properties
- Solubility
- Soluble in
- Water (at 1%)
- Nutritional Information
- Key Properties
- Fully characterized
- High-purity Reb A
| Value | Units | Test Method / Conditions | |
| Added Sugars | 0 | g/100g | — |
| Ash Content | 0.02 | g/100g | — |
| Calcium Content | 10 | mg/100g | — |
| Calories* | 0 | kcal/100g | — |
| Cholesterol Content | 0 | mg/100g | — |
| Dietary Fiber | 0 | g/100g | — |
| Iron Content | 0 | mg/100g | — |
| Moisture Content | 3.36 | g/100g | — |
| Potassium Content | 0 | mg/100g | — |
| Protein Content | 0 | g/100g | — |
| Saturated Fat | 0 | g/100g | — |
| Sodium Content | 14 | mg/100g | — |
| Total Carbohydrate** | 0 | g/100g | — |
| Total Fat | 0 | g/100g | — |
| Total Sugars | 0 | g/100g | — |
| Trans Fat | 0 | g/100g | — |
| Vitamin D | 0 | mcg/100g | — |
Regulatory & Compliance
- Certifications & Compliance
- Allergen Status
The product is in accordance with Food Allergen Labeling and Consumer Protection Act (FALCPA), Annex II of Regulation (EU) n° 1169/2011 on food information to consumers, and Health Canada, the Canadian Food Inspection Agency (CFIA).
- Regulatory Status
- United States:
- Truvia® Stevia RA95 has the status of Generally Recognized As Safe (GRAS), in accordance with United States Food and Drug Administration (US FDA) regulations for use as a direct food substance, when used in accordance with Good Manufacturing Practices (GMPs).
- Truvia® Stevia RA95 is produced in accordance with current food Good Manufacturing Practices (GMPs) under a comprehensive Hazard Analysis and Critical Control Points (HACCP) program and in compliance with applicable parts of 21 CFR, Part 117 of the Code of Federal Regulations.
- Truvia® Stevia RA95 complies with the FAO/WHO JECFA monograph (73rd JECFA, published in the FAO JECFA Monographs 10, 2010) for steviol glycosides, including Rebaudioside D.
- Canada:
- Truvia® Stevia RA95 is an approved food additive per the listing of steviol glycosides from Stevia rebaudiana Bertoni in Health Canada’s List 9. List of Permitted Sweeteners.
- Truvia® Stevia RA95 complies with the Joint FAO/WHO Expert Committee on Food Additives (JECFA) specifications for steviol glycosides (73rd JECFA, published in the FAO JECFA Monograph 10, 2010).
- Mexico:
- AGREEMENT determining the additives and adjuvants in foods, beverages and food supplements, their use and sanitary provisions. DOF 16/07/2012. Annex VII
- European Union:
- Truvia® Stevia RA95 complies with all applicable EU laws, in particular with Regulation (EC) 1333/2008 on food additives and Regulation (EU) 1131/2011/EC on the approval of steviol glycosides.
- Truvia® Stevia RA95 complies with Regulation (EU) 2016/1814 amending Annex to Regulation 231/2012 laying down specifications for food additives listed in Annexes II and III to Regulation 1333/2008 of the European Parliament and of the Council as regards specifications for steviol glycosides (E960).
- United States:
- Applicable Certifications
- Certified Kosher by the Orthodox Union (OU)
- Certified Halal by the Islamic Food and Nutrition Council of America (IFANCA)
Technical Details & Test Data
- Overall Quality Rating of Lemon Lime CSD Sweetened with RA95 versus ViaTech™ TS300+
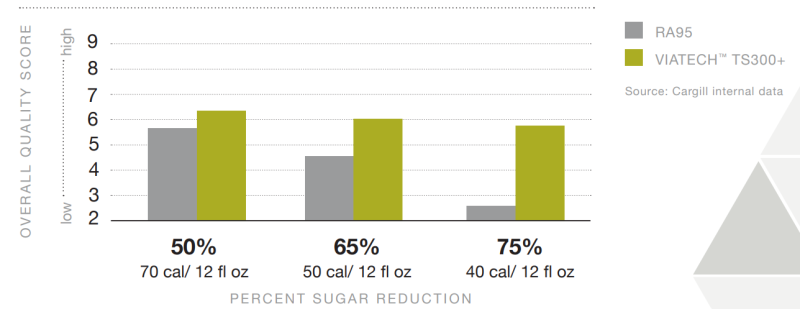
- Quantitative Descriptive Analysis Results
RA95 versus ViaTech™ TS300+ (500 ppm in water)
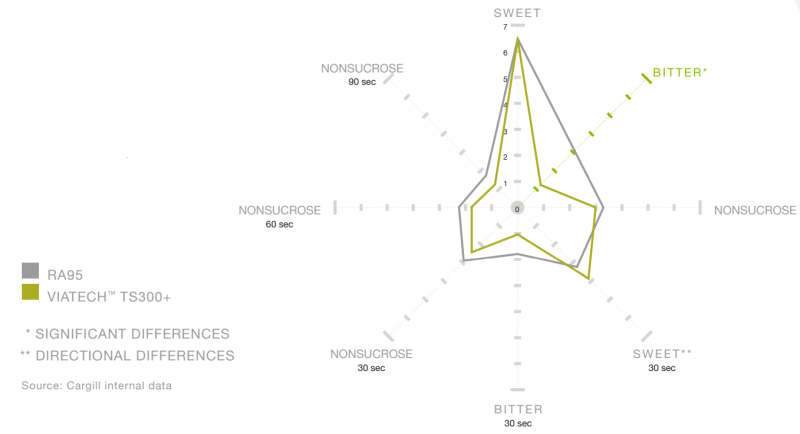
* * Significant Differences
** Directional Differences.ViaTech™ TS300+ significantly reduces bitterness compared to RA95 at higher usage levels, overcoming undesirable off-notes.
Packaging & Availability
- Regional Availability
- Packaging
2 x 10kg foil bag in a box
Additional Information
- Material Numbers:
100010257 - 2 x 10 kg foil bag - SAP Material Name:
TRUVIA® STEVIA RA95
- Material Numbers:
Storage & Handling
- Shelf Life
- 1,095 days
- Storage/Shelf Life
Product should be stored in a cool, well ventilated and dry area at ambient temperature and humidity and away from odors and chemicals.
The recommended best when used by date for Truvia® Stevia RA95, under these conditions and in original unopened packaging is 1,095 days from the date of manufacture. Product stored beyond the best when used by date should be evaluated periodically for fitness of use.