Knowde Enhanced TDS
Identification & Functionality
- Animal Feed & Nutrition Functions
- Technologies
- Product Families
Features & Benefits
- Animal Feed & Nutrition Features
- Product Background
- No doubt, mycotoxins present one of the biggest issues in modern animal farming. Recent evidence suggests that even low levels of multiple mycotoxins, under long-term field exposure, can impact animal performance and health. This negative, synergistic additive impact is exacerbated when combined with other stress factors, typically seen in real farming production, such as climatic conditions, stock density, poor quality of feed, low level of (subclinical) infections, etc.
- To contextualize: the cumulative impact of the long-term exposure to several mycotoxins (even at a low level) in the same farm, may be completely different between winter and summer. We thus miss the bigger picture. That means we are in need of comprehensive technologies, with multiple modes of action and proven efficacy in vivo. Comprehensive technologies can help the animal confront several stress factors under the prism of concomitant mycotoxin exposure, even if the latter is considered low.
- As such, Escent® S is a pioneering technology that helps animals cope with both abiotic and biotic stressors via a multifaceted, holistic approach. In vivo data back-up the five modes of action Escent® S.
Applications & Uses
- Markets
- Animal Species
Properties
- Physical Form
Technical Details & Test Data
- Modes of Action
- Supports the functions of the liver and the kidneys
- Prevents oxidative damage
- Stimulates the animal's immunity
- Triggers biotransformation and detoxification processes within the liver
- Adsorbs & binds polar (water soluble) toxins
- Reducing Systemic Exposure to Mycotoxins
Previous in vitro testing has proven the great ability of Escent® S to bind a great number of mycotoxins. However, in vitro results do not warrant an equal level of efficacy in vivo. For this reason, Innovad® in collaboration with Gent University (Belgium), developed and validated a ‘biomarker’ method that detects mycotoxins and their (phase I and phase II) metabolites in the blood of chickens and pigs. The validated method was, then, deployed for the evaluation of the in vivo detoxification efficacy of Escent® S, with very promising results. The research revealed that Escent® S exerted a significant detoxifying ability and reduced the systemic exposure of multiple mycotoxins in both species.
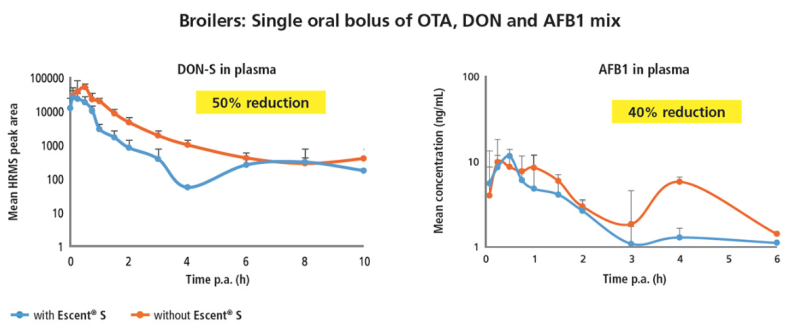
The findings themselves are particularly important, however, the real breakthrough has been the shift away from a simplistic toxin-binding approach. To this, under the Escent® S treatment even non-polar mycotoxins (like zearalenone) were systemically reduced. The evaluation of commercial technologies against several mycotoxins and their in vivo detoxification efficacy remains, thus, key.
- Mycotoxin Detoxification Power Under Real Farming Conditions
The detoxification efficacy of Escent® S was evaluated under real poultry farming conditions with the analytical precision of Myco-Marker®.
Two groups were tested: The first group was a Competitor technology (1kg/T) which served as the Control group (current standard farm practice), and the second group was Escent® (1kg/T). The real mycotoxin risk and the true impact on animal performance and health status were bio-monitored at several time-points with the analytical precision of Myco-Marker®.
Summary of the main findings:
1. Escent® S reduced drastically the systemic exposure to mycotoxins when compared to the Competitor technology
- Although mycotoxin presence in feed was slightly higher in the Escent® S group, the level and presence of mycotoxins in blood were significantly higher in the Competitor (Control) group at the end of the trial. Namely, 60% of animals from the Competitor (Control) group were systemically exposed to mycotoxins whereas, only 10% of animals consuming Escent® S were systemically exposed to mycotoxins.
- Four (4) mycotoxins were detected in the Competitor (Control) group at the end of the trial whereas, only one mycotoxin was detected in the Escent® S group (deoxynivalenol; similar levels between the Escent® S and the Competitor (Control) group).
2. Escent® S reduced mortality: The study revealed that the mortality at the end of the trial was higher in the Competitor (Control) group (1.65%) than in the Escent® S group (1.10%).
3. Escent® S reduced sub-clinical symptoms and improved animal performance:
- The litter was wetter and the ammonia smell was stronger in Competitor (Control) group throughout the trial.
- A low level of fecal wet droppings were observed in the Competitor (Control) group but none in the Escent® S group.
- Relative increased prevalence of late, low-level coccidiosis in the Competitor (Control) group.
Competitor (Control) group (1kg/T) Escent® S group (1kg/T) Feed Risk Level A. Presence and concentrations of mycotoxins in Feed 1. Fumonisins (200 ppb) 1. Fumonisins (500 ppb) 2. Deoxynivalenol (260 ppb) Blood B.Relative Percentage of animals systemically exposed to mycotoxins (blood) 60% of animals 10% of animals C. List of mycotoxins present in Blood 1. Deoxynivalenol 1. Deoxynivalenol 2. Aflatoxins 3. Tenuazonic acid 4. Beauvericin Note: Similar levels (traces) of deoxynivalenol were detected between the two groups Competitor (Control) group (1kg/T) Escent® S group (1kg/T) Overall risk Heatmap Risk Scale Moderate Low Performance and Animal Health Status D. Mortality 1.65% 1.10% E. Animal Performance & Health indicators 1. Wetter litter throughout the trial 1. Less wet litter throughout the trial 2. Stronger ammonia smell 2. Reduced ammonia smell 3. Relative increased prevalence of late, low-level coccidiosis in the Competitor (Control) group 3. Relative reduced prevalence of late, low-level coccidiosis in the Competitor (Control) group Note: Caecum autopsy was performed in isolated cases (birds) with bloody diarrhea from both groups at the end of the trial (D= 60). The autopsy indicated a certain level of sub-clinical gut inflammation with lesions and hemorrhages indicative of late coccidiosis (Eimeria tenella), Iikely predisposed by mycotoxins. The field trial under a non-controlled environment confirmed the in vivo mycotoxin detoxification and stress-relief power of the Escent® S Technology.
At the same time Myco-Marker® proved a reliable and powerful biomonitoring tool in terms of animal exposure to mycotoxins: Myco-Marker® was deployed over the course of 30 days, demonstrating it can be easily used in field conditions to evaluate precisely stress impact in livestock.
Overall, the results of the field trial in a non-controlled environment demonstrated the power of the RISE® platform – a real breakthrough in the Mycotoxin-related stress control management.