Knowde Enhanced TDS
Identification & Functionality
- Technologies
- Components
- Esterified Butyrins
- Sorbic Acid
- Medium Chain Fatty Acids (Capric, Caprylic and Lauric Acid)
- Calcium Propionate
- Essential Oils
- Plant Extracts
- Natural Plant Gum
- Carrier
- Anticaking
Features & Benefits
- Animal Feed & Nutrition Features
- Product Background
- The increasing animal genetic potential puts significant stress on the animal's digestive system, often leading to a suboptimal digestive process and nutrient absorption. The negative impact of sub-optimal feed utilization has a significant impact on the economic viability of the farm. Butyric acid is an important dietary component that can help balance and stimulate a healthy intestinal microflora. Complementary to this beneficial molecule, essential oils, plant extracts, and specific fatty acids support the natural symbiosis between host and microflora under stressful conditions.
- Gut health/Intestinal health is the most determining factor for animal health in general, herd performance and eventually farm profitability. Pathogenic bacteria may colonize the gastrointestinal tract and result in clinical and sub-clinical disease. Reduced feed intake and daily gain, inactivity and decreased social interactions are all observed in animals with bacterial infections.
- Livestock producers are often forced to make use of unconventional protein and carbohydrate sources or materials of lower quality. This means that animals are constantly challenged with changing diet compositions and quality, which cause risks for their health.
- Stress often leads to health challenges and intestinal disorders and is linked with the ‘leaking gut’ syndrome. The end-product is subclinical inflammation which can cost up to 30% of the animal’s energy requirements.
- Regulation, food safety and animal welfare are also setting up new trends towards limitations or restrictions of antibiotic usage for disease treatment, medicated feed, zinc oxide etc.
Applications & Uses
- Markets
- Applications
- Use Level
- 0.25 - 3 kg/MT (Poultry), 0.5 - 3 kg/MT (Rabbits), 1 - 4 kg/MT (Fish grower feed), 0.5 - 3 kg/MT (Pigs), 2 - 2.5 kg/MT (Fish starter and pre-grower feed), 10 g/head/day (Calves)
Properties
- Appearance
- Free flowing powder
- Physical Properties
- Note
Possibility to go up to 1% Dosage, only after contact with your local expert.
| Value | Units | Test Method / Conditions | |
| pH (in 10% Solution) | 5 - 6 | — | — |
| Specific Gravity | 0.55 - 0.75 | kg/l | — |
| Moisture Content | max. 7.5 | % | Karl Fischer Method |
| Butyric Acid Content | 10 - 12 | % | — |
Technical Details & Test Data
- Average NIR Spectrum
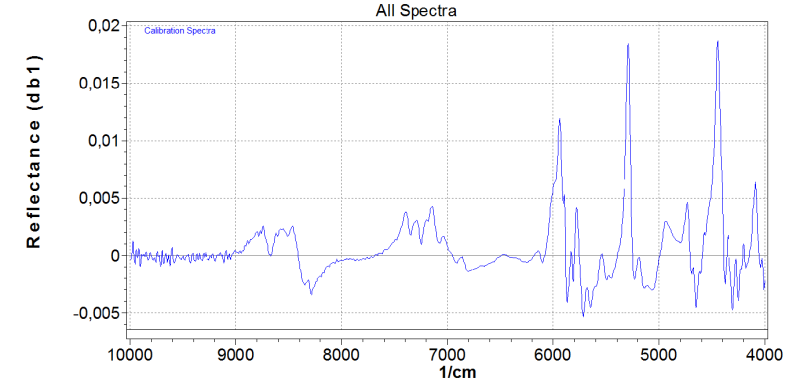
- Modes of Action
1. Reinforce gut integrity
- Stimulates the growth of villi
- Reinforces the intestinal defense
- Enhances the intestinal barrier
2. Reduce inflammatory response
The anti-inflammatory properties of butyric acid are complemented by plant extracts rich in alkaloids and essential oils found in the unique Lumance® recipe. Their efficacy and synergistic effect were demonstrated both in vitro and in vivo.
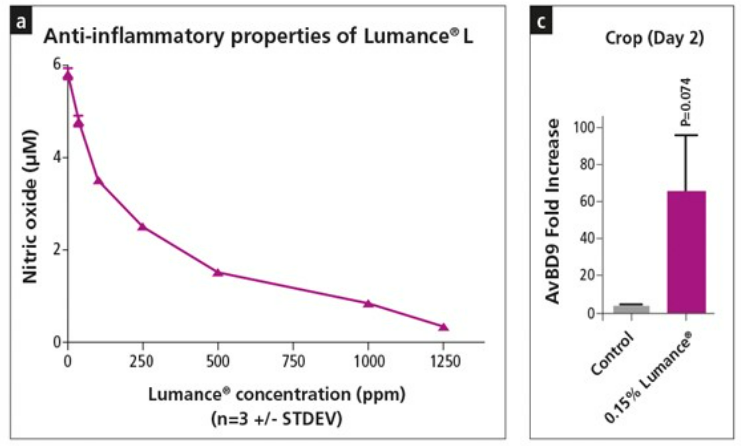
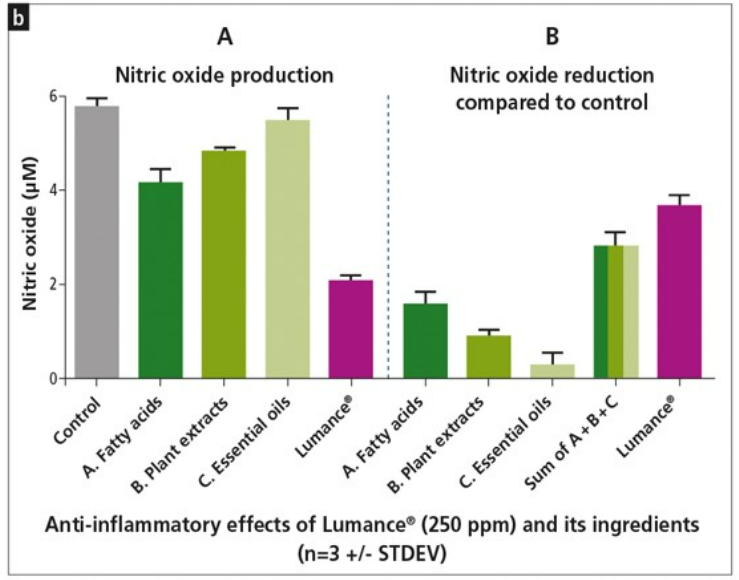
a) In vitro inhibition of LPS induced inflammation in macrophage producing cell lines by Lumance® expressed as reduction of Nitric Oxide,
b) Demonstration of the synergistic effect of Lumance®
c) In vivo demonstration of the anti-inflammatory effect of Lumance® (gene expression of the chicken Host Defense Peptide, β-defensin 9 - AvBD9)
3. Balance the lumen and its gastrointestinal microflora
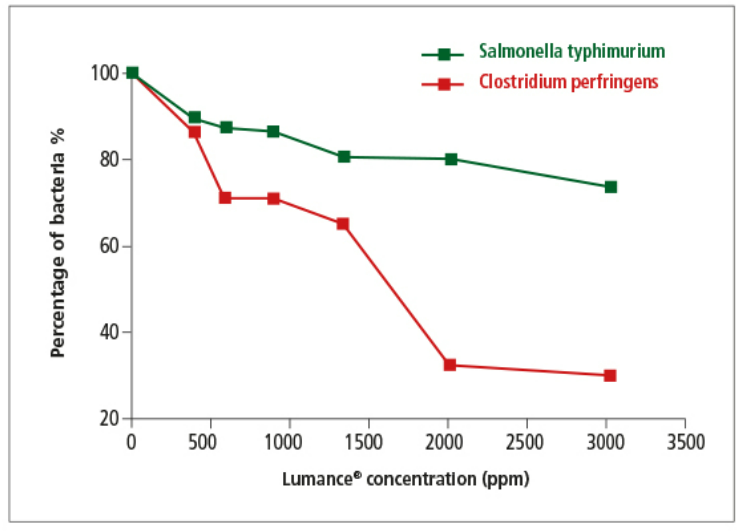
Lumance® has been carefully designed to reduce the impact of pathogens and support the growth of beneficial bacteria. The graph below shows the direct antibacterial effect of Lumance in vitro against a) Clostridium perfringens and b) Salmonella Typhimurium
4. Protect against ROS
Butyrate along with the plant extracts and essential oils added in Lumance® have been selected for their antioxidant activity and oxidative stress inhibitory effect.
- Effectiveness for Intestinal Health and Antibiotic Replacement
- Anti-inflammatory activity of Lumance®
First, Innovad® set up in-vitro and in-vivo experiments in collaboration with the university of Ghent (UGent) and Oklahoma (OSU) to evaluate the anti-inflammatory activity of Lumance®.
This research showed that Lumance® exerts a superior anti-inflammatory effect (in a dose response manner) against gut epithelial cells challenged with bacterial enterotoxins. This is of great importance as recent findings demonstrate that the control of pathogens at subclinical level is only a minor mechanism of Antibiotic Growth Promoters (AGPs) and that reduction of inflammation is indeed the main mode of action behind the growth promoting activity of AGPs.It is worth noting that in these tests Lumance® outcompeted the anti-inflammatory activity of oxytetracycline (OTC), the benchmark used for decades in the field. Similar beneficial activity has been demonstrated in vivo with a number of anti-inflammatory biomarkers such as C-reactive protein and Host Defense Peptides.
- Synergistic effect of Lumance®
It is believed that a combination of different compounds that provide multiple modes of action may hold the biggest promise as antibiotic alternatives in animal feed for four major reasons:
1) Single antibiotic alternatives fail to meet the sum of the beneficial effects of antibiotics
2) A possible synergistic effect from different alternatives. This may lead to reduction of the required effective dosage.
3) The gut integrity can be strengthened in the presence of specifically selected alternative compounds reducing thus, the ‘leaky gut syndrome’ and cases of diarrhea and wet litter.
4) The host immune response may be enhanced with an integrated approach.
Despite the growing body of evidence on improvement of overall animal performance with the combined use of more than one NAGP ingredients, the existing literature on the ability of these combinations to suppress the inflammatory response is relatively scarce. We here report the synergistic anti-inflammatory activity of Lumance®, as evaluated in a well-established gut epithelial cell model. The data shows that the anti-inflammatory effect of Lumance® is superior to any of its single components (fatty acids, plant extract and essential oils). In fact, the anti-inflammatory activity of Lumance® was 25% higher than the theoretical sum of its components.- Lumance®: Strengthening the gut integrity
Researchers also demonstrates that Lumance® enhances significantly the tight junctions even under bacterial enterotoxin (LPS) challenge in a well-established model used for the study of intestinal barrier functions (Fig3). This is of particular importance, taking into account that several stressors including poor diet (high in anti-nutritional factors, rancidity etc.), pathogens and toxins can increase the gut permeability leading often to chronic inflammation and disease such as Necrotic Enteritis in poultry. In simple words, it is proven that Lumance® can improve cases of ‘leaky gut syndrome’, diarrhea and wet litter.
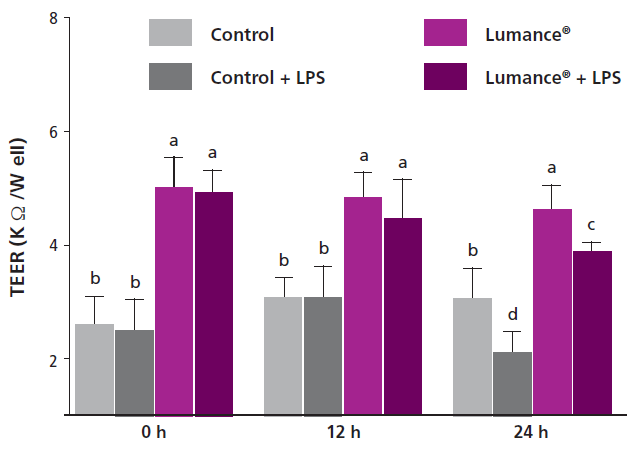
Fig. Effects of Lumance® and LPS on intestinal barrier integrity measured by TEER in IPEC-J2 cells. The intestinal cells were incubated for 5 days in case of Lumance® prior to challenging with LPS on day 5 post-differentiation; TEER was measured at 0, 12, and 24 h after LPS challenge, respectively. Values are means ± SD, n = 3. Different superscript letters (a, b, c, d) indicate statistically significant differences of mean values, P < 0.05.
- Lumance®: Prevention of necrotic enteritis
A study was conducted with the university of Ghent (UGent) which a) evaluated the in-vitro antibacterial activity of Lumance® and b) correlated the findings with a large-scale in-vivo trial that measured the ability of Lumance® to replace three commercial non-antibiotic feed additives used to control necrotic enteritis (NE) and maintain performance in the absence of antibiotic growth promoters.
The in-vitro research showed that Lumance® effectively inhibited the growth of C. perfringens in a dose-response manner.
The in-vivo trial confirms those results. It shows that Lumance® successfully controlled necrotic enteritis and replaced effectively all other additives, without the use of medication and growth promoters with a substantial cost saving potential.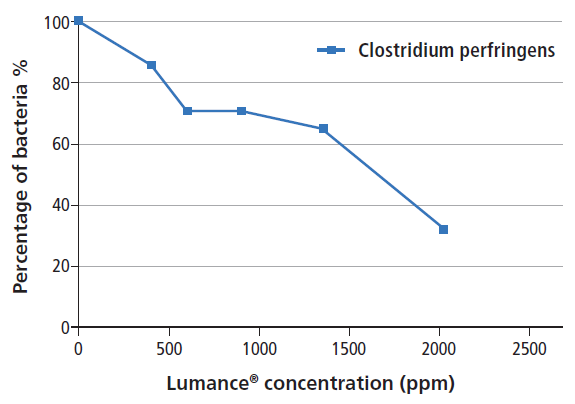
Fig. Trial results of Lumance® against C. perfringens
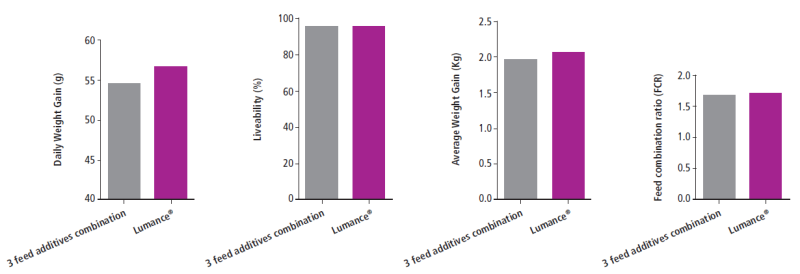 Fig. Effect of the experimental diets on the performance indices of broilers at 36th day
Fig. Effect of the experimental diets on the performance indices of broilers at 36th dayEO No1 EO No2 EO No3 (Oregano) EO No4 Mean % St. Dev. Mean % St. Dev. Mean % St. Dev. Mean % St. Dev. Lumance® 1 min at 90°C 101 7.7 93.9 7.3 94.9 6.6 101.1 8.6 Lumance® 5 min at 90 °C 97.7 2 98.9 2.2 94.9 1 99.5 1.5 Lumance® 1 min at 120°C 96.7 2.1 96.7 3.3 92.3 3.1 98.3 2.8 Lumance® 5 min at 120°C 101.5 6.7 101.6 3.9 99 4.9 105.4 7.3 Table 1. Mean values and standard deviation (n=3) of the percentage (%) recovery of the essential oils (EOs) from three different batches
of the Lumance® Technology, after heat treatment at different temperatures and times.- Lumance®: Proven slow and targeted release in the gut
Lumance® has been tested in an advanced in-vitro model mimicking the digestion of products in the gastro-intestinal tract (stomach, duodenum and hind gut). The sophisticated three-step enzymatic incubation proved the slow and targeted release of the n-butyric acid which is protected in the Lumance® platform in its esterified Technology. In simple terms, it was proved that a) the esterified n-butyric acid within Lumance® by-passes the stomach with practically no losses, b) it gets activated only at the duodenum level and, c) it reaches the hind gut.
- Need for rigorous field data
Lumance® has been used successfully in different geographies, farming conditions, animal species (including aqua), genetics, climates and legislation frameworks in terms of use of AGPs. Just an example in order to contextualize; to our knowledge, Lumance® has proved until now the only effective program for simultaneous replacement of both ZnO and antibiotics (neomycin), used traditionally to prevent post-weaning diarrhea in pigs. It is estimated that more than 10 million animals have been treated in Spain for simultaneous replacement of neomycin and ZnO and have been reared successfully in real large-scale farming conditions, whilst improving their performance.
- Lumance®: natural vs synthetic
In the recent years, the Feed Additives Market has been flooded by myriads of blends comprising (wholly or partially) of plant-based (inspired?) ingredients; both plant extracts or essential oils. However, the origin, quality and consistency of such materials, most of the times, remain unclear (‘natural’, ‘natural-identical’, natural-like’, ‘extra natural’ etc.). We guarantee that the oregano within the Lumance® Technology originates from 100% natural Greek oregano (Origanum vulgare hirtum) which results in superior anti-inflammatory activity when tested against synthetic carvacrol (data to follow).
To this, Innovad® has put in place a thorough (in house), Analytical System to screen:
a) the authenticity and,
b) the biological activity of active ingredients.- Lumance®: ‘formulation’ properties
Lumance® has undergone rigorous testing with practically no losses during pelleting and extrusion processes for both its essential oils and the fatty acids.
In conclusion, feed additives or better said in-feed technologies can be a good alternative to antibiotic replacement only when supported by rigorous data (in vitro and in vivo):
- For each animal species and condition tested,
- Data that elucidate their exact mode of action,
- Data that prove their thermal as well as shelf-life stability
- Anti-inflammatory Alternative to Antibiotics
Aiming to find an effective alternative to antibiotics, research groups at university of Ghent (UGent) and Oklahoma (OSU) set up in-vitro and in vivo experiments to evaluate the anti-inflammatory activity of Lumance®. Earlier, it was proposed that Antimicrobial Growth promoters (AGPs) can directly reduce inflammation through inhibition of cytokines production by inflammatory cells, thus saving energy for growth. The inflammatory response shares various molecular mediators and signaling pathways that can ultimately lead to decreased growth performance and endangered health in poultry and other production animals.
- Effective alternative to antibiotics
Effective alternatives to AGP can be chosen based on known anti-inflammatory properties or by specific in vitro tests. Among the proposed anti-inflammatory alternatives to antibiotics, plants extract and fatty acids are composed of wide variety of compounds, which are a good source of anti-inflammatory agent. A combination of different alternatives to antibiotic these two compounds may hold the most promising method to substitute antibiotics in animal feeds. In the present study, it is hypothesized that the commercial mixture of plant extract, essential oils, and fatty acids (Lumance®) would be synergistically effective in the reduction of inflammation in an in vitro and in vivo model.
Lumance® (obtained from Innovad®, Belgium) is a complex combining slow release and protection technologies ensuring that medium and short chain fatty acids, esterified butyrate, essential oils, anti-inflammatory compounds and polyphenols are delivered in a gut active way for a powerful and effective anti-inflammatory activity.
- Anti-inflammatory activity of Lumance®
I. In vitro trial
The anti-inflammatory activity of Lumance® was measured by the production of nitric oxide (NO) in an in vitro model using the RAW 264.7 assay, essentially as described by Wu et al (2003). In this in vitro inflammation model, Lumance® inhibited LPS-induced nitric oxide production with an IC50 value of 1471 (95 % CI 716, 2226) mg/l. The inhibition of NO production was not the consequence of cell death because it was established earlier using the tetrazolium salt WST-1 method that cell viability was not affected in any of the treatments.
II. In vivo gene expression trial
180 1-day-old male Cobb broilers were obtained from a commercial hatchery (Siloam Springs, AR, USA) and grown over a 10-day experimental period. The experiment was carried out in 20 pens with 9 broilers per pen. After 3 days of acclimatization, all birds were fed a non-medicated corn-soybean basal mash diet supplemented with or without Lumance® for 7 days.
On days 2 and 4 birds from each treatment were sacrificed with 1 bird/pen. A segment of the crop and jejunum was harvested from each bird and snap frozen in liquid nitrogen for RNA extraction and subsequent real-time PCR analysis of the expression levels of the chicken ≤-defensin 9 (AvBD9) gene.- Capabilities of HDPs
HDP are immunomodulatory molecules that have evolved to provide broad range of protection against a variety of pathogenic microbes. They are critical components of the innate immune system. HDPs also inhibit LPS induced pro-inflammatory cytokine production such as TNFα, IL-6, IL8 and IL-10. The overall consequence of HDPs function is the control of inflammation along with maintenance of the immune responses required for resolution of infections. Real-time PCR results indicated that, relative to control on day 2, Lumance® increased AvBD9 gene expression in the crop by 65-fold, but the induction was almost reduced to the basal level on day 4.
Lumance® showed the highest AvBD9 induction in the crop is likely due to the synergistic effect between short- and medium-chain fatty acids and other plant extracts. However, it is unknown why the ability of the product to induce AvBD9 subsided in the crop by day 4. Although the HDP induction is expected to reduce in the lower GI tract as butyrate gradually gets absorbed and metabolized, it is surprising to see no obvious effect on AvBD9 gene expression in the jejunum on either day 2 or 4.
- Promoting gut health and performance
Nevertheless, the HDPs that are induced and released in the upper GI tract by butyrate products are expected to exert a profound impact on the microbiota composition in the lower GI tract, which will in turn alter nutrient digestion and utilization and subsequent gut health and growth performance.
Taken together, supplementation of Lumance® enhanced the HDP gene expression in the GI tract of birds. These products have potential to replace antibiotics for disease control in prevention. However, the product showed the strongest capacity to reduce nitric oxide (NO) production in vitro and to induce HDP expression in the crop and, therefore, have beneficial impact on gut health and reducing inflammation.
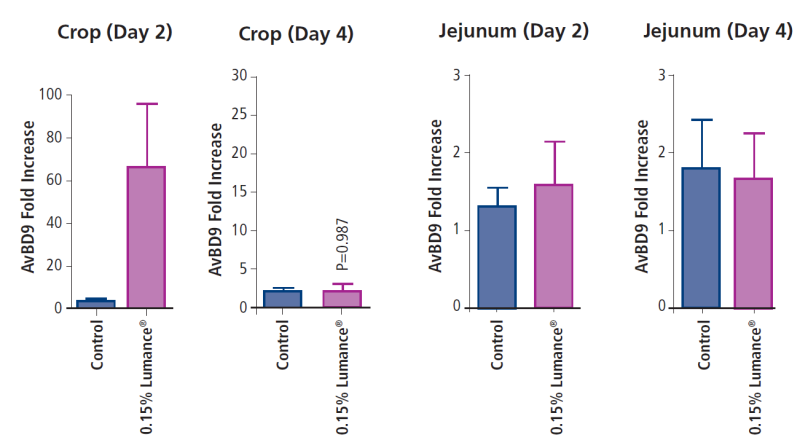
Figure. Effect of Lumance® on AvBD9 gene expression in the GI tract of broilers.
Packaging & Availability
- Packaging Type
- Packaging Information
- Standard 25 kg export worthy bags on wooden fumigated pallets.
- Other net weights (1 kg*, 5 kg*, 10 kg, 20 kg, 750-1000 kg, bulk) on demand.
*Marked unit packs are packed in an outer carton up to max. 25 kg.
Storage & Handling
- Shelf Life
- 2 Years
- Storage Conditions
24 months when stored in a cool, well ventilated and dry environment out of direct sunlight in unopened packing.