Knowde Enhanced TDS
Identification & Functionality
- Ingredient Name
- Food Ingredients Functions
- Pharma & Nutraceuticals Functions
- Ingredients
- Chitosan
Features & Benefits
- Benefit Claims (Health)
- Food Ingredients Features
- Product Highlights
- Safe and non allergenic : 100% animal-free, GMO-free, allergen-free
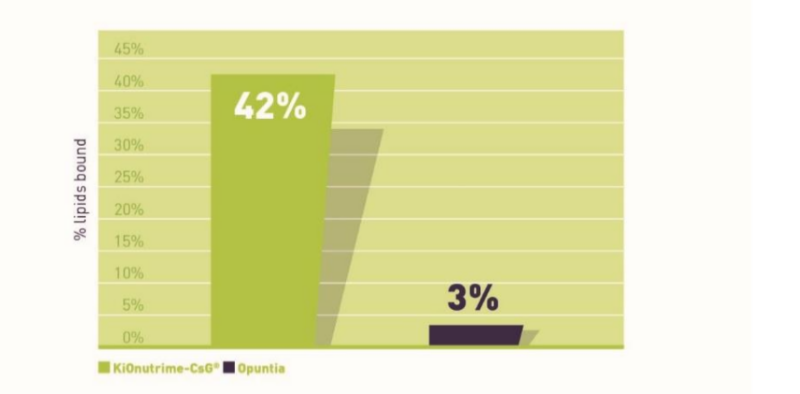
- Superior fat-binding capacity: binds up to 42% of dietary fats(1)
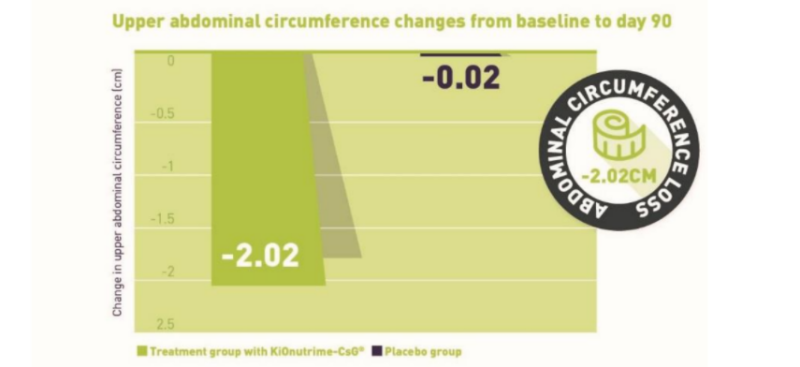
- Clinically proven weight loss up to -5.9kg and an abdominal circumference loss up to -8cm(2)
- Generally Recognized as Safe (GRAS) pursuant to 21 CFR §173.280
- EFSA claim – Maintenance on normal LDL Cholesterol
- High purity ingredient – Made In Belgium!
- Odorless & tasteless
- Appropriate for use in Kosher, Halal and vegetarian products
Applications & Uses
- Markets
- Food & Nutrition Applications
Regulatory & Compliance
- Certifications & Compliance
- EUROPEAN UNION
Substantially equivalent to shellfish chitosan according to Novel Food Regulation
(EC) 258/97 – article 4.3 with regard to:- Composition
- Nutritional value
- Metabolism
European Food Safety Authority (EFSA) 5th batch released late June 2011
Positive evaluation related to chitosan and maintenance of normal blood LDL- cholesterol.
In weighing the evidence, a meta-analysis of Randomized controlled trials, which investigated the effects of chitosan consumption on blood lipids, has been evaluated by the Panel and showed a statistically significant reduction in total and LDL-
cholesterol concentrations.3g of chitosan should be consumed daily.
- UNITED-STATES
Generally Recognized as Safe (GRAS) pursuant to 21 CFR §173.280
Section 8(413) (c) of the Dietary Supplement health and Education Act (DSHEA) of 1994 defines a “new dietary ingredient” as a dietary ingredient that was not marketed in the United States before October
15, 1994.Chitosan is listed on the following lists as dietary supplement ingredients use before 94
- National Herbal Products Association (NNFA)
- Council for Responsible Nutrition (CRN)
- Utah Natural Products Association (UNPA)
FDA bioterrorism registration number: 19190952740
Technical Details & Test Data
- Mechanism
In acidic stomach KiOnutrime® CsG is protaonated and able to bind negative charged molecule (fats, fatty acids, Bile salts) through electrostatic interaction .

- Superior Fat-Binding Capacity
- Clinical Study
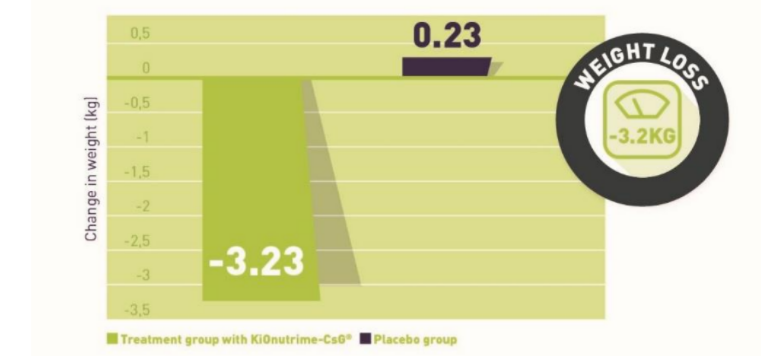
Single-blind, placebo controlled randomized clinical study of chitosan for body weight reduction; Vandit R Trivedi, Ph D; Milan C Satia; Audrey Deschamps; Véronique Maquet; Ronak B Shah; Padmanabh H Zinzuwadia; Jayesh V Trivedi; Nutritional Journal; Submitted; 2016.
Indication studied: treatment of excess weight, weight control
- Study medication: KiOnutrime-CsG®
- Dose: 5 capsules containing 500mg of chitosan a day
- Subject population: 86 male or female subjects within 18-65 years of age and with BMI
- between 26 and 35
- Duration: 90 days
- Results: -3.2kg and -2.02cm of abdominal circumference
A change in weight up to -5.9kg
A change in abdominal circumference up to -8cm