Knowde Enhanced TDS
Identification & Functionality
- Chemical Name
- Agrochemical Functions
- CASE Ingredients Functions
- Cleaning Ingredients Functions
- Cosmetic Ingredients Functions
- Industrial Additives Functions
- CAS No.
- 164462-16-2
- EC No.
- 423-270-5
- Technologies
- Product Families
- Chemical Name
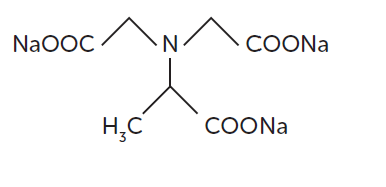
- Methylglycine N,N-diacetic acid, trisodium salt
- Molecular Weight
- 271.1
- Chemical Structure
Features & Benefits
- Labeling Claims
- Agrochemicals Features
- HII Features
- Product Features
- Dissolvine M-X granules are free-flowing, odorless and have good storage stability at 65% Relative Humidity and 40°C. The fact that MGDA granules are crystalline contributes to storage stability. In a storage test for 6 months at 40°C and 65% relative humidity, no ammonia like smell was noticed. Caking of Dissolvine® M-X under these conditions is negligible when the relative humidity is 65% or lower. Dissolvine® M-X when mixed with bleaching agents will remain color stable even after several months’ time.
- The bulk-density of the granules (min 700 kg/m3) ensures that tablet size and pods containing MGDA have a good size or volume. The particle size distribution of the Dissolvine® M-X granules provides non-dusting behavior. The particle size distribution makes MGDA granules easily compatible with other ADW ingredients and tableting when needed can take place with little effort. The low friability of Dissolvine® M-X is another positive aspect in handling this product.
- These functionalities of Dissolvine® M-X in combination with the high assay / high chelation capacity are favoring MGDA as the chelate of choice.
- The thermal stability of Dissolvine® M-X is excellent; this is reflected by the auto ignition temperature being > 400°C. In handling solids like Dissolvine® M-X dust explosion properties are also important to know. The Lower Explosion Limit (LEL) of MGDA-Na3 solid according to test standard EN 14034-3 was measured at our safety laboratory and the result of 250g/m3 shows a rather high value for a dust explosion due to combustion.
- Benefits of Chemical Structure
- MGDA has three carboxylic groups and, together with the central nitrogen atom, these carboxylic groups provide strong multiple bonds with di- and trivalent metal ions. The small molecular size enables rapid action at low temperatures and short contact time. The MGDA trisodium salt is fully REACH registered.
- The excellent low toxicity and low eco-toxicological profile allow for non-dangerous labeling. MGDA-Na3 is listed on EPA’s Safer Chemical Ingredients List in the United States.
- MDGA is a strong chelate for hard water and transition metal ions. Using MGDA as an ingredient in cleaning formulations improves the descaling and cleaning capabilities. This includes whiteness and color care benefits in laundry and stain removal abilities in automatic dish washing (ADW). Since MGDA is also a strong chelating agent for heavy metal ions, such as Fe and Cu, it enhances product stability and prevents negative effects of transition metals.
- Product Overview
- Dissolvine® aminopolycarboxylate-based chelating agents are used in countless applications to control metal ions in water-based systems. They are highly effective for controlling water hardness ions as well as for cleaning surfaces, descaling boilers, processing textiles and preventing scale formation.
- When it comes to controlling metal ion reactivity, Dissolvine® chelating agents are an important tool for reducing the detrimental effect of metal catalysts in peroxide cleaners and in pulp bleaching for paper manufacturing. Other applications include improving personal care formulations and stabilizing the bleaching process in textile.
- Finally, Dissolvine® chelating agents are also used extensively to enhance the chemical and physical properties of metal ions, ranging from metal plating, providing essential elements to growing plants and supplying iron for H2S gas scrubbing.
- Innovating and supplying high performing products with a low environmental impact is important for Nouryon. In our search for a product that delivers excellent chelating performance with readily biodegradable properties, Nouryon introduced Dissolvine® M-40. The active component is MGDA, a chelating agent that has a proven track record in many different institutional and household cleaners. Today, in addition to Dissolvine® M-40, another MGDA product is offered to the market; Dissolvine® M-X, a free flowing granule.
- MGDA is a fast-working, strong builder with excellent ecological properties, being readily biodegradable and not labeled as dangerous. This makes MGDA an ideal replacement for ingredients under regulatory pressure, such as phosphates (which are banned in various regions due to water eutrophication) in automatic dish washing (ADW), in car care and laundry. It is a drop-in replacement for NTA in industrial and institutional cleaners. In cleaning applications, Dissolvine® M-40, as well as Dissolvine® M-X, will outperform widely used builders like phosphates, citrates, gluconates and zeolites due to their stronger bonds with hard water ions.
Applications & Uses
- Applications
- Applicable Processes
- Application Technique
- Home Care Applications
- I&I Cleaning Applications
- Industrial Additives End Use
- Applications
- In household cleaning, Dissolvine® M-X effectively replaces phosphorous containing builders in dishwashing detergents. It enhances the cleaning power of a cleaner / detergent by sequestering hard water ions from water and dirt. It prevents the deactivation of surfactants from hard water metal ions.
- It also deactivates transition metal ions thus stabilizing detergents that contain peroxides. In industrial & Institutional cleaning, Dissolvine® M-X forms stable, water-soluble metal complexes with all potentially harmful metal ions
- It dissolves existing and prevents new scales from causing problems in water circulation equipment (in e.g. the power, brewing, and sugar industries). Because of its low molecular weight this chelate performs very well in short contact cleaning.
- Dissolvine® M-X is also very suitable as a metal carrier, a stabilizer of process baths and for neutralizing trace impurities. It can be used in e.g. alkaline Zinc and electroless Copper plating.
Properties
- Typical Properties
- Rheometric Properties
- Note
* Based on Fe-sequestering capacity
| Value | Units | Test Method / Conditions | |
| NTA-Na3 | max. 0.20 | wt% | — |
| Active ingredient* | min. 81 | wt% | — |
| Density | min. 700 | kg/m3 | — |
| Auto Ignition Temperature | min. 400 | °C | — |
| Solubility in water | 1100 | g/l | — |
| Molecular Mass | 271.1 | — | — |
| Value | Units | Test Method / Conditions | |
| pH | 11.5 | — | — |
Regulatory & Compliance
- Certifications & Compliance
Technical Details & Test Data
- Test Results
Solubility
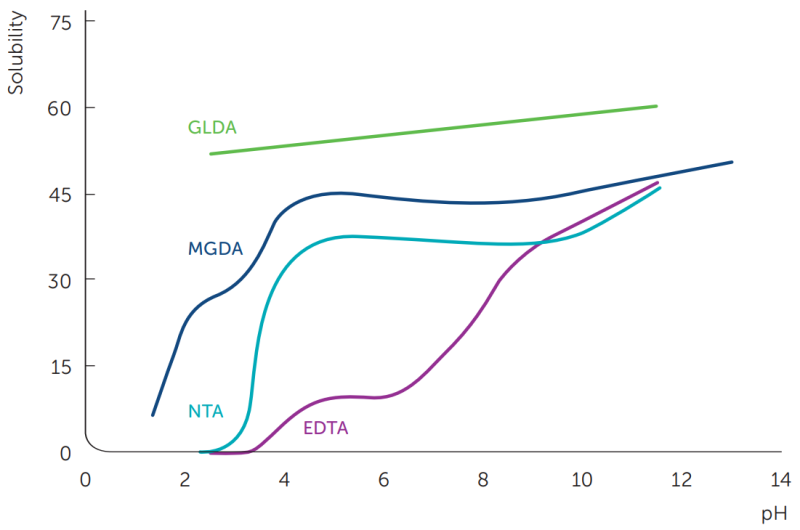
The solubility of MGDA as a function of pH is shown in figure 2. Like most aminocarboxylic chelates, the solubility is greatest for the fully ionized form that is present at high pH, quite similar to that of NTA. The solubility of MGDA is surpassed by the extraordinary high solubility of GLDA (Dissolvine® GL) across the entire pH range.
Table 2 lists the solubility of several chelates in various media. Here too the solubility of MGDA is similar to NTA, which may enable Dissolvine® M-40 to be used as a direct replacement for NTA in many formulations. Unlike NTA, Dissolvine® M carries no hazard warnings and may also qualify for eco labeling.
Table 2: Solubility of several chelates in various media at 25°C
MGDA NTA EDTA GLDA NaOH, 15% ~ 20 ~ 23 ~ 20 ~ 60 NaOH, 28% ~ 3 ~ 7 ~ 6 ~ 53 Acetic acid, 28% ~ 7 ~ 1 < 1 > 50 HCl, 28% ~ 6 ~ 13 < 1 > 50 Ethylene glycol ~ 26 low low ~ 45 Figure 2: Solubility of chelating agents, expressed as their sodium salt, in water at various pH levels
Density
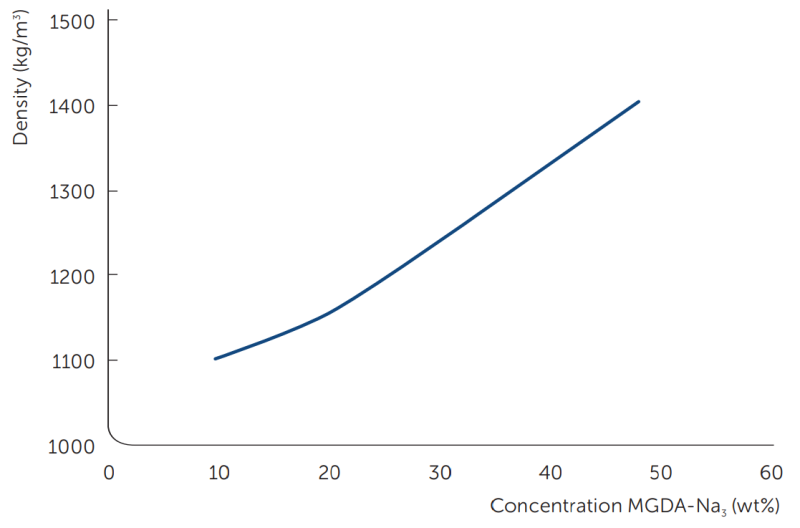
The density of the liquid can be used as a quick reference for checking the concentration of the material (figure 3). The density of the solid is important for packaging and plays a role in tableting the granules.
Chemical Stability
Like all the Dissolvine® chelating agents,The thermal stability of Dissolvine® M-S has been determined using Thermal Gravimetric Analysis (TGA) and Differential Scanning Calorimetry (DSC). MGDA-Na3 crystals will lose all crystal water at temperatures around 200°C and start to decompose at temperatures above 300°C.
Solutions of MGDA-Na3 are fully stable at temperatures of up to 170°C for six hours, or at 150°C for one week. This means that MGDA can be a useful biodegradable alternative to EDTA when used for scale prevention or for cleaning boilers.
Figure 3: Density of a solution of MGDA-Na3 plotted against concentration
Acid/base Dissociation Constants
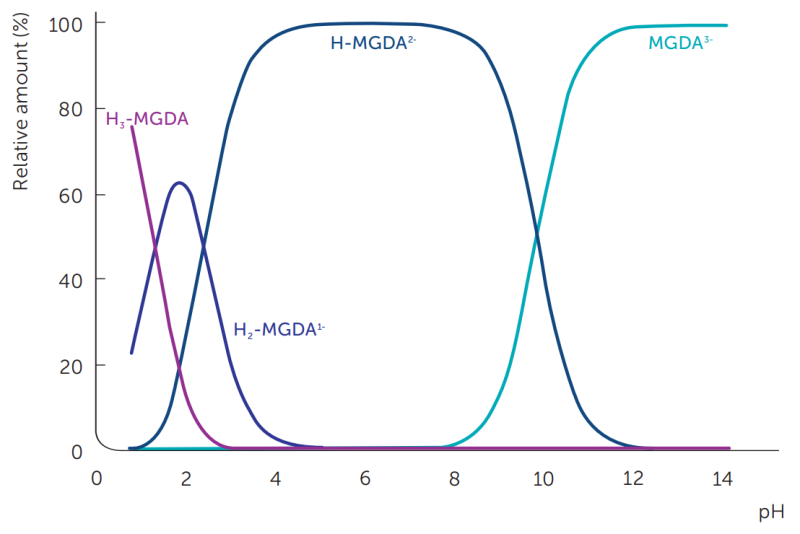
Dissolvine® chelating agents are amino polycarboxylic acids that ionize in water to multiple charged species depending on pH. The ionization constants, or pKa values, for MGDA are shown in table 3. Again we see a close similarity to NTA. The ion species distribution of the MGDA molecule as a function of the pH can be calculated from the pKa values (figure 4).
Chelating Power
Chelating agents are added to products or processes to control the properties of metal ions. For example, chelating agents are used in cleaning and personal care to complex with cations (e.g. Calcium, Magnesium, Fe, etc.) and prevent reactions with other ingredients that often lead to precipitation. In other applications, chelates are used to remove unwanted scale by complexing the scale metal ions.
Table 3: The acid dissociation constants (pKa)* for MGDA, NTA and EDTA
MGDA NTA EDTA pKa1 9.9 9.7 10.2 pKa2 2.6 2.5 6.2 pKa3 1.5 1.8 2.7 pKa4 not available 1.0 2.0 pKa5 not applicable not applicable 1.5 pKa6 not applicable not applicable 0.0 *A.E. Martell, R.M. Smith, NIST Critically selected stability constants of metal complexes (NIST standard reference database 46, Version 7.0, 2003). pKa values: as determined at an ionic strength of 0.1M and at a temperature of 25°C, or if not available at 20°C.
Figure 4: Ionized forms of MGDA as a function of pH
Chelates are used in copper and nickel plating to deliver metal ions in the ideal form for the plating process. For each application, it is important to select a chelating agent that is sufficiently strong to do the job. An indication of the chelates’ strength or affinity for a certain metal ion can be derived from the dissociation constants, stability constants and conditional stability constants.
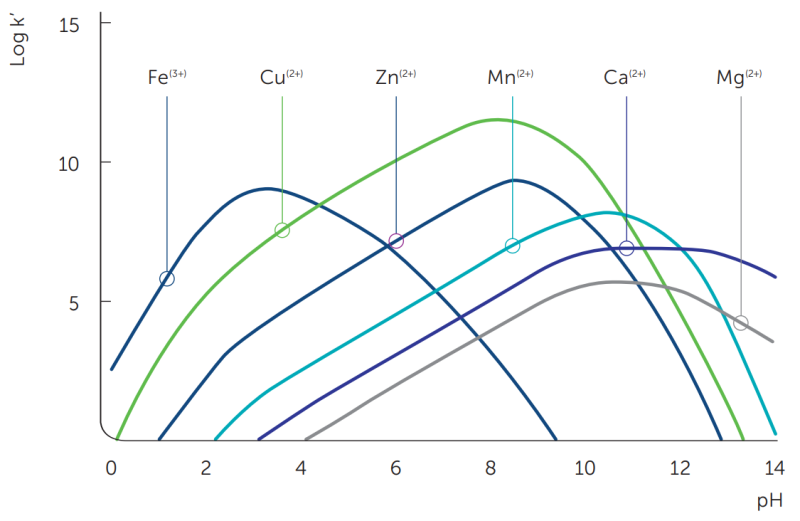
The stability or equilibrium constant (K), generally expressed as log K, is an indication of the strength of the complex formed between the metal ion and the chelating agent. The higher the log K value, the tighter the bond between the metal ion and the chelating agent, which in turn increases the likelihood that a complex will be formed (table 4).
The pH of the system and the oxidizing nature of the environment can affect the stability and effectiveness of the chelating system. For each metal complex, there is an optimum pH and an active pH range in which the metal complex is stable. The conditional stability constant is an indication of the stability of the complex as a function of the pH (figure 5).
Chelating Capacity
Chelates generally form 1:1 complexes with metal ions. The quantity of chelating agent needed depends both on the concentration of metal ion to be chelated and the molecular weight of the chelate. In general, while a chelate with a high molecular weight will complex a metal ion more strongly than a chelate with a low molecular weight, a larger quantity will be needed. The chelating capacity of Dissolvine® M-40 and Dissolvine® M-S expressed as mg chelate/g MGDA product are compared to NTA and EDTA products in table 5.
The experimentally determined CaCO3 chelating value (CaCV) of Dissolvine® M-40 is 147 mg/g and 297 mg/g Dissolvine® M-S. These measurements were performed using Ca2+ as a titrant and with two different means to detect the endpoint: one with a Ca2+ ion selective electrode and another using carbonate as a precipitation indicator. The found values correspond well with the theoretical CaCV.
Table 4: Stability constants (log K values1) and active pH range for Dissolvine® M-40 / Dissolvine® M-S (MGDA)
Metal ion Ca2+ Cu2+ Fe3+ Mg2+ Mn2+ Zn2+ Log K 7.0 13.9 16.5 5.8 8.4 11.0 Active pH range2 6 - 14 1 - 11 0 - 8 7 - 11 4 - 11 2 - 11 1. A.E. Martell, R.M. Smith, NIST Critically selected stability constants of metal complexes (NIST standard reference database 46, Version 7.0, 2003); Log K values as determined at an ionic strength of 0.1M and at a temperature of 25°C or 20°C. Log K for Fe3+ and Mn2+ the figure was extracted from P.T. Anastas, Green Processes, Volume 9: Designing Safer Chemicals.
2. Active pH range: calculated for demineralized water at 0.1 mol/l. Lower pH limit: the conditional stability constant logK’ ≥ 3. Upper pH limit is based on the precipitation of the metal hydroxide; at upper pH limit the fraction chelated ≥ 95%.Unlike very strong chelates like EDTA and DTPA, the ‘chelating ability’ of MGDA is dependent on the testing conditions (the indicator, temperature and concentration). Besides the theoretical chelating capacity, there is also a practical ‘chelating capacity’. For example, when using Ca ions this practical chelating capacity is often called Ca dispersing power.
To illustrate the strong calcium binding strength of MGDA, experiments have been performed with various chelating agents and the calcium ion indicator Hydroxy Naphthol Blue (HNB), which is used in this experiment as a competitive chelating agent. HNB has a relatively high affinity for calcium and shifts color from blue to red when fully complexed to calcium. As a result, the color of a solution containing calcium ions, HNB and the tested chelate gives a measure for the calcium binding efficiency of the chelate vs. the HNB.
Figure 5: Theoretical curves of the conditional stability constant (log K’) of MGDA for various metal ions as a function of pH (1:1 metal:chelate complex).
In figure 6 the calcium affinity at pH 11–12 for a number of chelates is compared. The key finding is that Dissolvine® M-40 / M-S as well as Dissolvine® GL are very effective for complexing hard water ions.
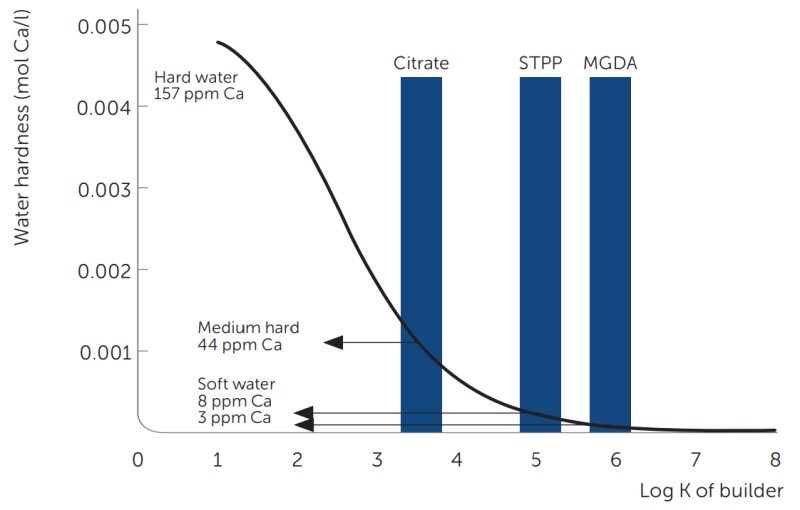
Another measure of the ability to complex the calcium and magnesium hard water ions, and thus to soften water, is presented in figure 7. It shows a calculated plot of water hardness versus the strength of a builder (log K) in the presence of an equal molar amount of Ca ions and chelates. MGDA is capable of achieving low water hardness levels, while citrate is only capable of providing a medium hardness unless a significantly higher amount is used vs. Ca ion present. The ideal wetting conditions for a fast cleaning process appear only at a low water hardness; a few ppm of Ca.
Table 5: Theoretical chelating capacity expressed as mg of chelated substance /g Dissolvine® M-40, Dissolvine® M-S, EDTA and NTA for several metal ions and CaCO3
Product Assay wt. % CaCO3 Ca2+ Cu2+ Fe3+ Mg2+ Mn2+ Zn2+ Dissolvine® M-40 40 147 59 93 82 36 81 97 Dissolvine® M-S 80 297 120 190 167 73 164 195 NTA-Na3 as 40 % solution 40 156 62 99 87 38 85 102 EDTA Na4 (Dissolvine® E-39) 39 103 41 65 57 25 56 67 Figure 6: The calcium complexing efficiency of various chelating agents in competition with Hydroxy Naphthol Blue (HNB) at pH 11–12
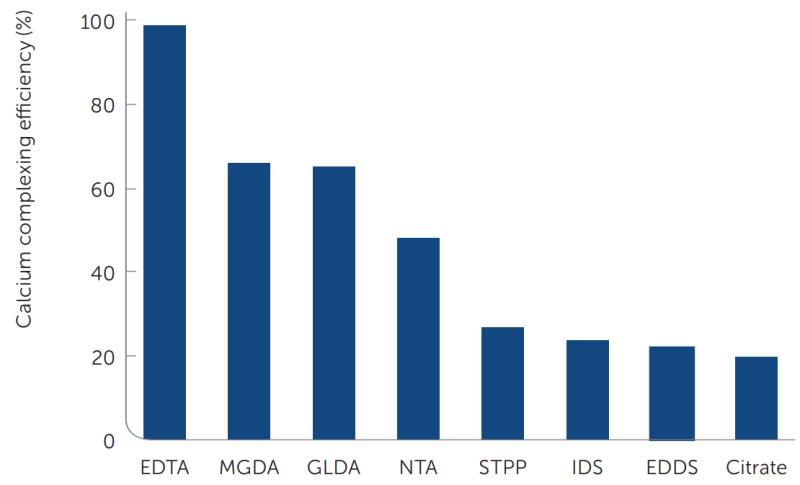
Figure 7: Water hardness reduction in the presence of various chelates versus Log K of the Ca-chelate stability constant.
Packaging & Availability
- Country Availability
- Regional Availability
- Regional Availability
- Africa, Asia, Asia Pacific, China, Europe, Global, India, Latin America, Middle East, North America, Oceania
Storage & Handling
- Storage Conditions
- Dissolvine® M-40 and Dissolvine® M-X are stable products under normal and recommended storage conditions. There are no decomposition or dangerous reactions known under normal conditions.
- Due to its high pH, Dissolvine® M-40 should be stored in containers made of corrosion-resistant material (e.g. stainless steel or plastic containers).
- Materials to avoid in storage containers: Aluminum, Zinc, Copper alloys, Copper, Nickel. Don’t combine MGDA with hypochlorite bleach. More information on handling and safety issues can be found in the safety data sheet of Dissolvine® M-40 and Dissolvine® M-X.