Knowde Enhanced TDS
Identification & Functionality
- Ingredient Name
- Food Ingredients Functions
- Pharma & Nutraceuticals Functions
- Ingredients
- Boswellia Extract, Boswellic Acid
Features & Benefits
- Labeling Claims
- Fast Facts
- Low, 100 mg/day dose
- Improved joint comfort in just 5 days
- 56% reduction in WOMAC scores from baseline at 30 days
- 69% reduction in WOMAC scores from baseline at 90 days
- 10 pre-clinical and clinical studies
- Sustainable, botanical ingredient
Applications & Uses
- Markets
- Applications
Regulatory & Compliance
Technical Details & Test Data
- Extensive Scientific Support for AprèsFlex®
AprèsFlex 5-Day Joint Support has been the subject of extensive pre-clinical and clinical studies to understand its mechanism of action and its efficacy in supporting joint comfort and mobility.
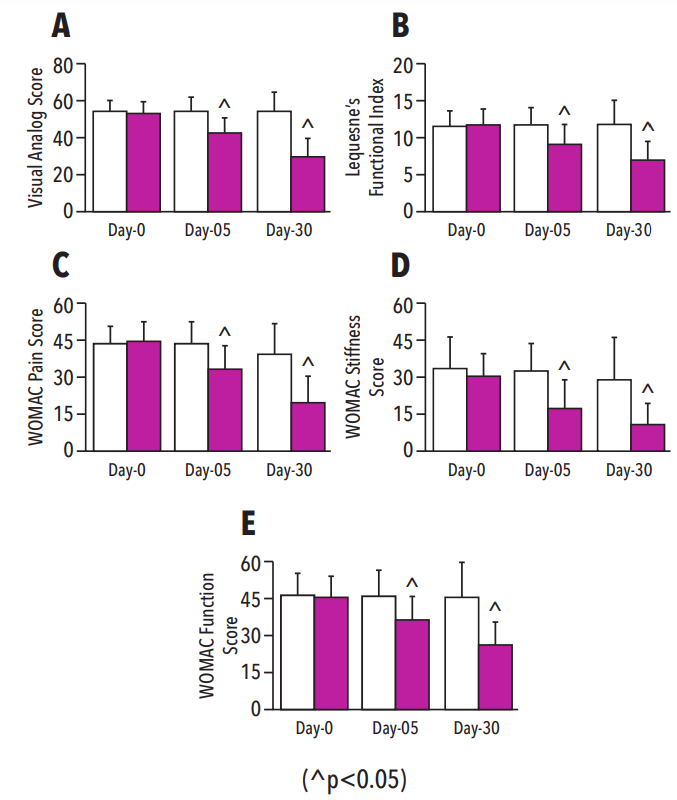
Karlapudi and colleagues examined the efficacy of AprèsFlex in the management of joint comfort and flexibility in a randomized, double-blind clinical trial.
Measurements included:
- Visual Analog Score (VAS) (Graph A)
- The Lequesne algofunctional index (LFI) (Graph B)
- The Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) (Graphs C,D,E)
- Various serum biomarkers (MMP3, TNF, hsCRP, COMP, CPII and C2C)
Measurements were done on Days 0, 5 and 30
The study showed that AprèsFlex:
- Provided significant reduction (p<0.05) in all the pain scores at five days compared to the placebo
- Provided significant reduction (p<0.05) in all function scores at five days compared to placebo
Previous studies with AprèsFlex showed 56% reduction in pain scores at 30 days. A similar study with AprèsFlex showed a 69% reduction in pain scores at 90 days.
Safety Studies
In Sprague Dawley rats, acute oral LD50 of AprèsFlex was determined to be > 5000 mg/kg. Acute dermal LD50 was > 2000 mg/kg. No changes in body weight or adverse effects were observed. In a subacute 28-day repeat dose toxicity study, the NOAEL was determined to be > 2500 mg/kg. No abnormal changes related to the study product were demonstrated in hematology, clinical chemistry, or histopathology. No adverse effects were observed.
Beyond joint comfort and flexibility improvements, AprèsFlex has also been shown to offer statistically significant impacct on biological markers associated with joint health and inflammation, including TNF-alpha, C-Reactive Protein (CRP), and Interleukin-6 (IL-6). It was also shown to significantly inhibit matrix metalloproteinase (MMP-3), an enzyme that breaks down cartilage, collagen, and connective tissue.
Sustainability
A 2022 3rd party audit of the Boswellia serrata gum resin used to make AprèsFlex based on a broad range of environmental, cultural and economic parameters concluded: “It is clear from the information collected from the stakeholders that [sustainability] is supported. Boswellia serrata has several sustainability advantages that prevents over-harvesting of PLT-sourced Boswellia compared to other sources and species of Boswellia.” PLT is committed to helping the communities where this ingredient is harvested thrive.