Knowde Enhanced TDS
Identification & Functionality
- Chemical Family
- Chemical Name
- Pharma & Nutraceuticals Functions
- CAS No.
- 9063-38-1
- EC No.
- 618-597-7
- Technologies
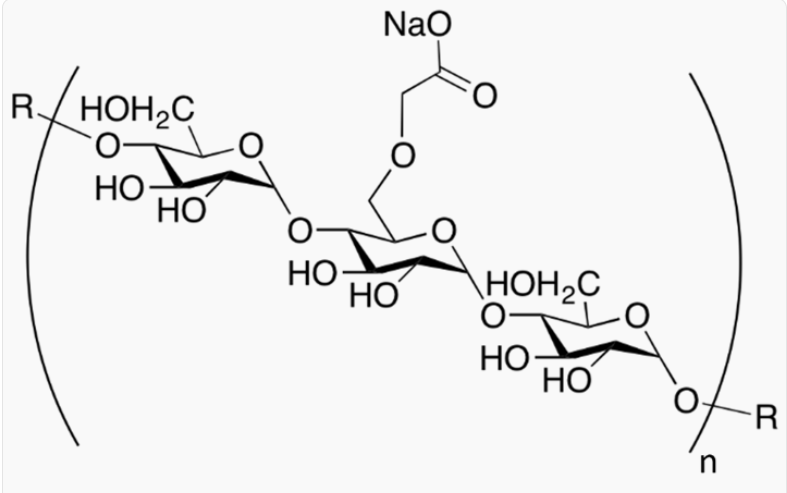
- Chemical Structure
Features & Benefits
- Benefit Claims
Applications & Uses
- Applications
- Application
- GLYCOLYS® sodium starch glycolate is a superdisintegrant for swallowable tablets, orally dispersible tablets and hard capsules. The usual concentration employed is between 2% and 8%
Properties
- Appearance
- White or almost white fine very hygroscopic powder
- Insoluble in
- Ethanol, Ether
- Dispersible in
- Water to form a viscous colloidal solution
- Typical Properties
- Physico-Chemical Properties
- Microbiological Values
- Note
- "ChP" stands for Chinese Pharmacopeia
- (*)-Compliance data - Tests not performed
- (**)-Monitoring plan
| Value | Units | Test Method / Conditions | |
| Acidity or Alkalinity (pH) | 5.5 - 7.5 | — | — |
| Average Mean Particle Diameter | 45 | µm | — |
| Brookfield Viscosity | max. 75 | mPas | — |
| Combined Sodium | 2.8 - 4.2 | % | — |
| Ethanol Content | max. 6.0 | % | — |
| Heavy Metals Content (*) | max. 0.002 | % | — |
| Identification Test 1 (*) | Complies | — | ChP |
| Identification Test 2 (*) | Complies | — | ChP |
| Particle Size (sieve, Residue on 105 Microns) | max. 2.0 | % | — |
| Sodium Chloride (on DS) | max. 6.0 | % | — |
| Sodium Glycolate Content | max. 2.0 | % | — |
| Water Content (LOD) | 6 - 10 | % | — |
| Value | Units | Test Method / Conditions | |
| Assay Content | 2.0 - 4.0 | % | — |
| Iron Content (*) | max. 0.002 | % | — |
| Loss on Drying | max. 10.0 | % | — |
| Value | Units | Test Method / Conditions | |
| Escherichia coli (**) | Not detected | per gram | — |
| Salmonella (**) | Not detected | per 10g | — |
| Total Aerobic Microbial Count | max. 1000 | CFU/g | Plate count |
| Total Yeasts and Moulds Count | max. 100 | CFU/g | — |
Regulatory & Compliance
- Certifications & Compliance
- Chemical Inventories
- Grade
- Conformity
- Conforms to the requirements of the current monograph
- Chinese Pharmacopeia (ChP) SODIUM STARCH GLYCOLATE Please contact us for any statement regarding compliance to the General Chapters (elemental impurities, residual solvents, organic volatile impurities, metal catalyst, metal reagent).
Technical Details & Test Data
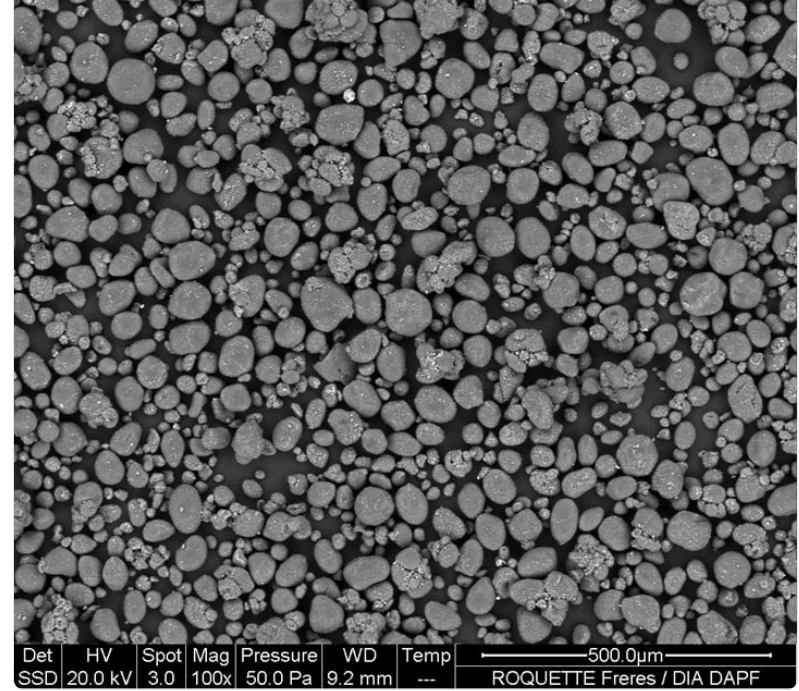
- Morphology
- Note
- Not intended for use in manufacture of parenteral dosage forms.
- Methods used by Roquette may be the Pharmacopeia methods or alternative validated methods which have been compared to the Pharmacopeia methods.
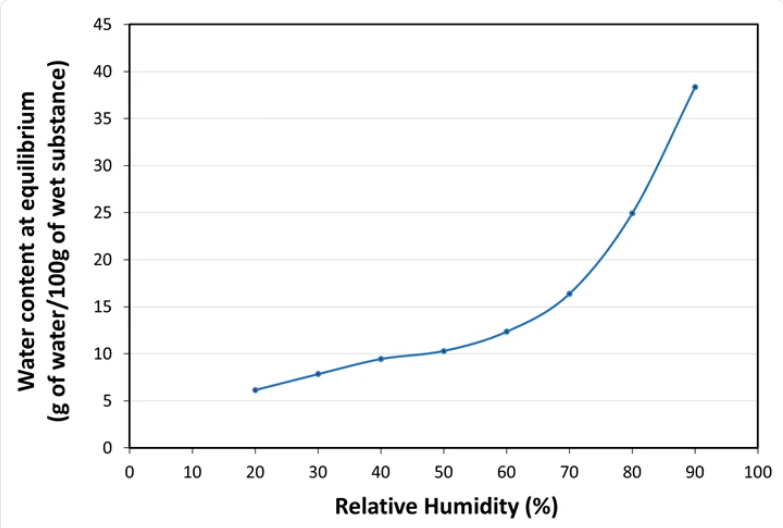
- Water Sorption Isotherm at 20°C
- Innovation Hub
- Innovation Hub by RoquetteLooking for technical support or formulation inspiration? Visit Roquette’s Innovation Hub.
Packaging & Availability
- Packaging Information
- ROQ Product Code: 731112
- Article (SKU) Code: 731112102H
- Package Size & Type: 50 kg lined fiber drum
Storage & Handling
- Storage & Shelf Life
- Expiry date Manufacturing date + 5 years, in its unopened packaging.
- The product durability may vary according to packaging type and manufacturing plant. Proper information is shown on labeling and CoA.
- We recommend to preserve the product in its unopened original packaging, preferably protected from wide variations of temperature and humidity.
- Upon opening, use the product as quickly as possible to prevent moisture regain.