Knowde Enhanced TDS
Identification & Functionality
- Chemical Family
- Chemical Name
- CAS No.
- 11097-59-9
- EC No.
- 234-319-3
- Technologies
Features & Benefits
- Labeling Claims
- Product Background
The collected and refined know-how to produce alumina hydrates via the sol-gel technique is used by Sasol to prepare other inorganic oxides or mixed oxides. The properties of these inorganic specialty materials are leading Sasol into new, interesting fields of research and application. One of these specialties is the hydrotalcite family (aluminum-magnesium compounds) which is obtained by hydrolysis of mixed alcoholates. Compared to alumina hydrates, hydrotalcites are even more alkaline in nature. Basicity is adjustable by increasing the Mg/Al ratio and/or incorporating anions other than OH-. Hydrotalcites have a double-layered metal hydroxide structure. The layers consist of magnesium and aluminum hydroxide octahedra sharing edges. Additional interstitial anions between the layers compensate the charge of the crystal and determine the size of the interlayer distance (basal spacing). While hydrotalcites are accessible through the corresponding metal salts, the metal alcoholate route, patented by Sasol, has advantages over other synthesis routes. Most important is that the Al/Mg ratio can be varied over a wide range. In addition, it is now possible to obtain hydrotalcites with a purity and a controlled anion content that have to date been unavailable.
Applications & Uses
- Markets
- Applicable Processes
- Base Chemicals End Uses
Properties
- Chemical Properties
- Physical Properties
- Particle Size Distribution
| Value | Units | Test Method / Conditions | |
| Magnesium Oxide : Aluminium Oxide Content | 30 : 70 | % | — |
| L.O.I. | max. 40 | % | — |
| Carbon Content | 0.5 - 3 | % | — |
| Silicon Dioxide Content | max. 350 | ppm | — |
| Ferric Oxide Content | max. 200 | ppm | — |
| Na, Ca, Ti each | max. 50 | ppm | — |
| Value | Units | Test Method / Conditions | |
| Surface Area | min. 250 | m2/g | — |
| Pore Volume | min. 0.5 | ml/g | — |
| Loose Bulk Density | 350 - 550 | g/l | — |
| Value | Units | Test Method / Conditions | |
| Maximum 25 μm | min. 20 | % | — |
| Maximum 45 μm | min. 40 | % | — |
| Maximum 90 μm | min. 85 | % | — |
Technical Details & Test Data
- Functional Materials for Corrosion Protection
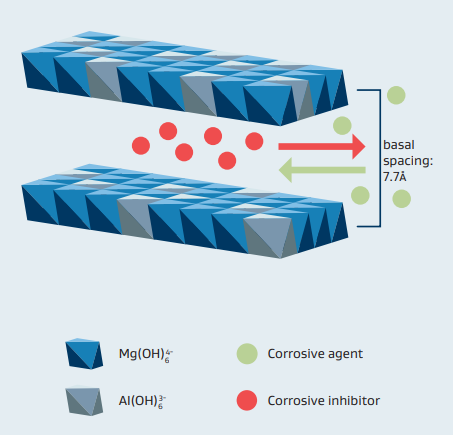
Layered double hydroxides of hydrotalcite structure are well known to be active in corrosion protection, especially in Chloride Induced Steel Corrosion in reinforced Concrete. This effect is largely due to their ability to act as anion exchange materials,
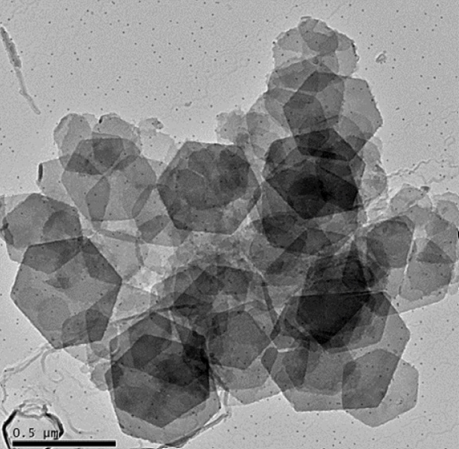
in which largely unwanted corrosives like chloride can be trapped inside the structure. Hydrotalcites consist of a layered structure where carbonate and water molecules are contained within two layers of magnesium and aluminum hydroxide octahedra sharing edgesHydrotalcites are produced by Sasol Germany GmbH under the tradename PURAL MG. This is done by their proprietary mixed metal alkoxide based sol-gel process which allows tailor made products of high purity. In contrast to alternative methods like precipitation, the Al/Mg ratio can be precisely adjusted over a wide range and unwanted anions are completely avoided. PURAL MG is easy to disperse into any formulation and can be applied as coating on steel.
Before being effective as corrosion inhibitor, PURAL MG must be activated. One effective way of doing this is by mild thermal treatment in order to remove carbonate ions. Additionally, carbonate ions can also be replaced by corrosion inhibitors like phosphates, nitrates or amino acids. If these hydrotalcites are incorporated into concrete or coated on steel, these inhibitors are released in a controlled manner and corrosive agents like Cl anions are trapped simultaneously inside the structure.
Packaging & Availability
- Country Availability
- Regional Availability