Knowde Enhanced TDS
Identification & Functionality
- Chemical Name
- Pharma & Nutraceuticals Functions
- CAS No.
- 9004-64-2
- EC No.
- 618-388-0
- Technologies
- Product Families
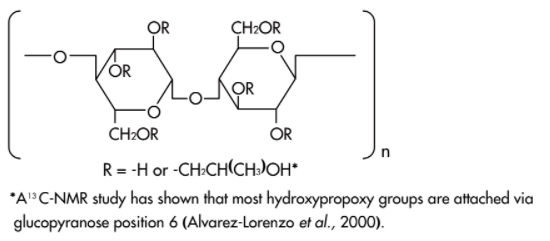
- Structure
Features & Benefits
- Labeling Claims
- Features
- Water insoluble, swells in water and works as dual functional ingredients, disintegrant and binder for tablets and pellets.
- Suitable grade can be selected depending on process and API characteristics.
- Non-ionic polymer which has less interaction with API and better stability.
Applications & Uses
- Markets
- Applications
- Manufacturing Technology
Properties
- Typical Properties
- Particle Size Distribution
- Note
*In-house Laser diffraction method
| Value | Units | Test Method / Conditions | |
| Chloride Content | max. 0.36 | % | USP NF |
| Heavy Metals content | max. 0.001 | % | USP NF |
| Hydroxypropoxy Content | 10.0 - 12.9 | % | USP NF |
| Loss on Drying | max. 5 | % | USP NF |
| pH | 5 - 7.5 | — | JP |
| Value | Units | Test Method / Conditions | |
| 90% Cummulative Particle Size | 100 - 150 | µm | Shin-Estu |
| Mean Particle Size | 45 - 65 | µm* | Shin-Estu |
Regulatory & Compliance
- Certifications & Compliance
- Chemical Inventories
- Grade
Technical Details & Test Data
- Anti-Capping Effect of L-HPC
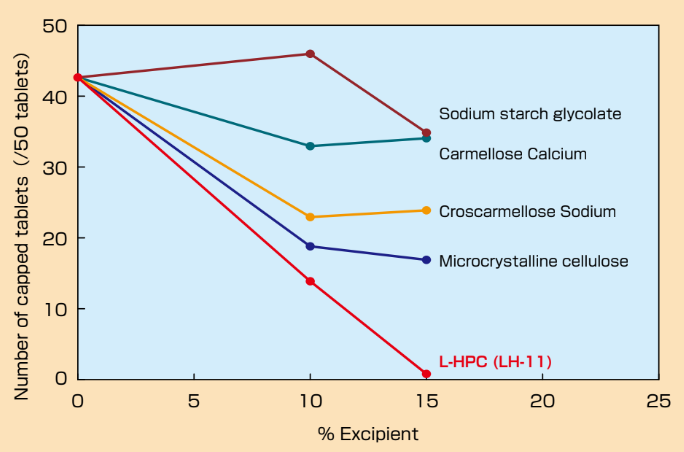
Ethenzamide tablets were prepared with various ratio of excipients and friability test was implemented in accordance with USP method.
- Pictures of Aspirin Tablets with Various Excipients (After stability test)
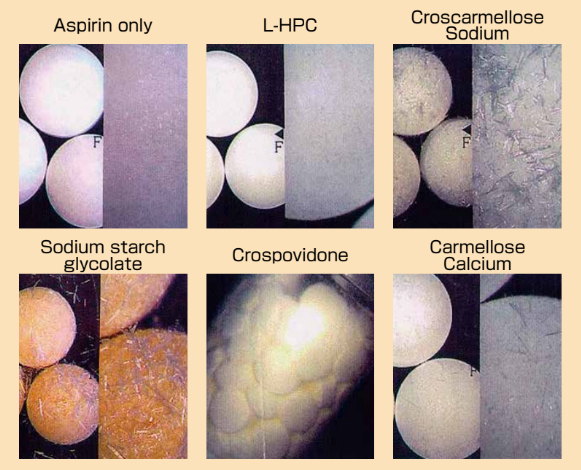
Aspirin tablets with 20% excipients were stored in closed plastic bottle at 50°C for 3 months.
Packaging & Availability
- Packaging Type
- Package
- 50 kg - Fiber drum with polyethylene double bag inside
- 1 kg - Polyethylene double bag
Storage & Handling
- Storage
Keep dry. Store away from excess heat and sunlight. Store in sealed containers.