Knowde Enhanced TDS
Identification & Functionality
- Chemical Family
- Ingredient Name
- Ingredient Origin
- Animal Feed & Nutrition Functions
- Food Ingredients Functions
- Pharma & Nutraceuticals Functions
- Ingredients
- Rhodiola Rosea Root Extract
- Technologies
- Product Families
- Chemical Composition
Roots and rhizomes of Rhodiola rosea contain phenylpropanoids and their glycosides (rosin, rosavin, rosarin), phenolic compounds (salidroside, tyrosol), flavonoids, terpenes (rosiridol, rosaridin, daucosterol, beta-sitosterol), antraglycosides, tannins (15.6%), organic acids - gallic, oxalic, succinic, citric, malic and substances of a lactone nature, essential oil and a large amount of manganese. The composition of the essential oil includes phenylethyl alcohol, P-phenylethyl acetate, cinnamaldehyde and citral.
Features & Benefits
- Benefit Claims
- Labeling Claims
- Product Background
Rhodiola rosea is one of the best plants providing adaptogenic properties. Rhodiola has been used since ancient times in Tibetan medicine. It grows in regions with a cold and temperate climate, like North America, the United Kingdom, and Ireland, in alpine mountain meadows (in the Alps, Pyrenees, Carpathians) and in the Pamirs. In Russia in the Urals, in the polar regions of Yakutia, in the mountainous regions of Eastern Siberia, Western Siberia and the Far East, on the coast of the White and Barents Seas.
The height limits of its spreading are 900–2800 m above sea level, most abundant at an altitude of 1700– 2000 m. Primary commercial thickets of Rhodiola Rose are in the highland regions of Buryatia, the Western and Eastern Sayan Mountains, as well as in the Kuznetsk Alatau".
- Features and Benefits
- GMO Free
- BSE/TSE free
- Allergen free
Applications & Uses
- Applications
- Food & Nutrition Applications
- Applications
Industry
- Cosmetic
- Pharmaceutical industry
- Food supplements
- Food and drinks
- Feeds
- Healthy nutrition
Properties
- Odor
- Characteristic
- Appearance
- Dark brown fine powder
- Residual Solvents (Ethanol)
- max. 5000
- Typical Properties
- Physico-Chemical Properties
- Microbiological Values
- Allergen Decleration EU
Allergens Direct Incorporation Cross Contamination Presence on the production line Presence on the production workshop Presence on the production factory Cereals containing gluten (1) and
products thereofNO NO NO NO Crustaceans and products
there ofNO NO NO NO Eggs and products thereof NO NO NO NO Fish and products thereof (2) NO NO NO NO Peanuts and products there of NO NO NO NO Soybeans and products there of
(3)NO NO NO NO Milk and products thereof
(including lactose) (4)NO NO NO NO Nuts (5) or products thereof NO NO NO NO Celery and products there of NO NO NO NO Mustard and products there of NO NO NO NO Sesame seeds and products
there ofNO NO NO NO Sulfur dioxide and sulphites at
concentrations of more than 10
mg/kg or 10 mg/l expressed as
SO2NO NO NO NO Lupines and products thereof NO NO NO NO Mollusk and product thereof NO NO NO NO (1) Cereals which contain gluten (i.e.w heat, rye, barley, oats, spelt, kamut or their hybridized strains) except: w heat-based glucose syrups including dextrose, wheat-based maltodextrins, glucose syrups based on barley, cereals used for making distillates or ethyl alcohol of agricultural origin for spirit drinks and other alcoholic beverages.
(2) Except: fish gelatine used as carrier for vitamin or carotenoid preparations, fish gelatine or singlass used as fining ag ent in beer and wine.
(3) Except fully refined soybean oil and fat, natural mixed tocopheols (E306), natural D-alpha tocopherol, natural D-alpha tocopherol acetate, natural D-alpha tocopherol succinate from soybean sources;vegetable oils derived phytosterols and phytosterolesters from soybean sources; plant stanol es ter producted from vegetable oil sterols from soybean sources.
(4) Except when used for making distillates or ethyl alcohol of agricultural origin for spirit drinks and other alcoholic beverages, lac tito.
(5) Almond (Amydalus communis L.) hazelnuts (Corylus avellana), walnut (Juglans regia), cashew (Anacardium occidentale), pecan nuts (Carya illinaiesis), brazil nut (Bertholletia excelsa), pistachio nut (Pistacia vera), macadamia nut and queensland nut
(Macadamia terniflora) and products thereof, except nuts used for making distillates or ethyl alcohol of agricultural origin for spirit drinks and other alcoholic beveragesIn accordance with the directive 1169/2011 EC.
- GMO Declaration
Present GM-Origin Cotton NO Not-Applicable Maize Yes (1) Non-GMO Potato NO Not-Applicable Rape NO Not-Applicable Soya NO Not-Applicable Sugarbeet NO Not-Applicable Tomato NO Not-Applicable Wheat NO Not-Applicable (1) From Maltodextrin
The following ingredients produced from genetically modified organisms are present in the product: None
In accordance with the regulation (EC) No 1829/2003 and (EC) No 1830/2003 of the European Parliament
| Value | Units | Test Method / Conditions | |
| Arsenic Content | max. 25 | ppm | ICP-MS |
| Cadmium Content | max. 1 | ppm | ICP-MS |
| Heavy Metals Content | max. 10 | ppm | ICP-MS |
| Lead Content | max. 3 | ppm | ICP-MS |
| Mercury Content | max. 0.1 | ppm | ICP-MS |
| Salidroside Content | max. 1 | % | HPLC Analysis |
| Total Rosavins Content | max. 3 | % | HPLC Analysis |
| Value | Units | Test Method / Conditions | |
| Ash Content | max. 5 | % | Ph.Eur./USP |
| Bulk Density | 0.4-0.7 | g/ml | Ph.Eur./USP |
| Loss of Drying | max. 5 | % | Ph.Eur./USP |
| Value | Units | Test Method / Conditions | |
| Enterobacteriaceae Content | max. 100 | CFU/g | Ph.Eur./USP |
| Escherichia Coli Content | 1 | gm | Ph.Eur./USP |
| Salmonella | 25 | gm | Ph.Eur./USP |
| Total Plate Count | max. 5000 | CFU/g | Ph.Eur./USP |
| Yeasts and Molds | max. 500 | CFU/g | Ph.Eur./USP |
Regulatory & Compliance
- Statements
- Non Irradiation/Non Ionized Statement
We hereby certify that the product has not been sterilized byionizing radiation at anypoint during the entire manufacturing process and therefore is in full compliance with the relevant legislated regulations (Directive 1999/2/CE). - BSE/TSE Statement
We hereby certify that the product contains no ingredients of ruminant origin and no materials derived from, or exposed to ruminants affected byor under quarantine for Transmitting Transmissible Spongiform Encephalopathy(TSE)/ Bovine Spongiform Encephalopathy (BSĖ) and it is conform to the EU legislation 999/2001. - Packaging Statement
We hereby certify that the packing used is in compliance with: Commission Regulation (EC)No 1935/2004 and Regulation (EU) No 10/2011 regarding packaging materials and articles intended to come into contact with food. - Pesticide Statement
We hereby certify, basis on our actual knowledge of production process, raw materials and equipment used potential pesticide residues in the above-mentioned product comply with the European legislation on pesticide residues, esp. Regulation (EC) No. 396/2005. - Residual Solvent Statement
We hereby certify that the product meets with: Directive E 2010/59/EU of 26 August 2010 amending Directive 2009/32/EC ofthe European Parliamentand of the Council on the approximation of the laws of the Member States on extraction solvents used in the production of foodstuffs and food ingredients. - Contaminant Statement
This is to certify that the product meets with:
REGULATION (EC) No. 1881/2006 and subsequent amendments as concerns the maximum level admitted of the following contaminants:- Aflatoxin B1
- Aflatoxins B1 + B2 + G1 + G2
- Ochratoxin
- Melamine
REGULATION (EC) No. 1933/2015 amending regulation (EC) No. 1881/2006 as regards maximum levels for polycyclic arom atic hydrocarbons, in particular:
- Maximum level of 10 ug/kg of benzo(a)pyrene
- 50 ug/kg for the sum of PAH4 (benzo(a)pyrene, chrysene, benz(a)anthracene and benzo(b)fluoranthene) in food supplements.
- Nanomaterial Statement
According to the definition of “nanomaterials" of the EU Regulation (EU) No. 1169/2011 of the European parliament and of the council of 25 October 2011 on the provision of food information to consumers, we hereby attest that no nanomaterials are used in the formulation or in the packaging material of the product. - Anti-Doping Statement
As per the list of 2017 and subsequent amendments of the world anti-doping agency, the ingredient is not a doping substance and not a combination of doping substances. The ingredient does not contain any doping substance. The ingredient does not result from a doping substance. - Expected Usage
The expected usage ofthis product is its incorporation as an ingredientin the food industryor the pharmaceutical industry.
- Non Irradiation/Non Ionized Statement
Technical Details & Test Data
- Plant Information
- Scientific name : Rhodiola Rosea L.
- Synonyms : Sedum roseum (L.) Scop.
- Botanical family : Crassulaceae
- Common names : Arctic root, golden root
- Geographical origin : Russian Federation, Siberia
HarvestPeriod (month/s or season) Spring / Autumn Method (manual/mechanical) Manual Used part Root and rhizomes Type of culture (wild/cultivated) Wild Plant growth stage Before / after flowering After Harvest
Crushed Yes Cleaned Yes Dried Electric Oven Additional Information
Treatments before/after harvest No / No Identification Botanical and chemical (Pharm. Russian Federation / USP monograph “Rhodiola rosea”) Storage In dry and cool well-ventilated place away from light - Extraction Details
Extraction method Solvent extraction Solvent(s) 70% Ethanol / 30% Water Drying method Vacuum impulse Carrier / excipient Maltodextrin (10 - 45%) Ratio (native) 4-5:1 Ratio (final) 2.5 - 3.5:1 - Product Flow Chart
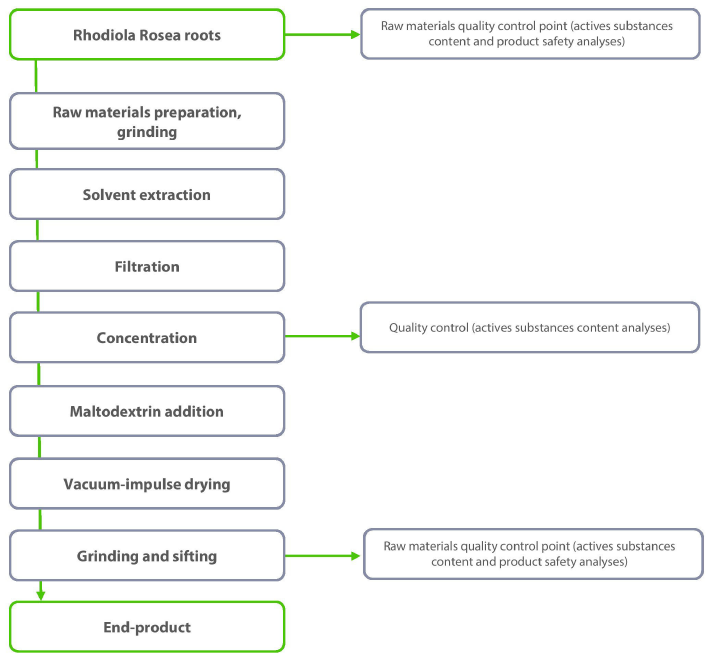
- Manufacturing Process
We have built a full production cycle - from the search and collection of raw materials, the manufacture of the product to the delivery of the order to the client's territory. This guarantees you stable prices, stable quality and continuous deliveries. We focus on the needs of our customers and, upon request, carry out additional analyzes, develop the necessary documentation. and we produce extracts and powders with the characteristics you need.
Verification : We check raw materials and final products with the help of laboratory tests at every stage from raw material preparation to finished extracts and powders. The supplied products comply with the EU safety and quality standards.
Safety & Health
- Influence
General Effectes on the Body
- Increases the body's resistance to adverse effects (environmental pollution, exposure to pathogenic microflora and viruses, exposure to high and low temperatures, toxic effects of ethanol)
- Improves physical and mental performance, contributes to the normalization of life after past diseases
- In patients with neurosis, when taking rhodiola, mobility of the inhibitory and excitatory processes is normalized
- Reduces fat accumulation with fatty tissue
- Reduces the risk of osteoporosis by increasing bone mineralization
- Protects nerve fibers and helps them recover from damage
- Lowers blood sugar levels in combination with cranberry
Stress
- Improves the appetite for the loss of hunger associated with stress (combination with hunter).
- Improves insomnia performance
- Increases the recovery function of the body after stress
For Male Health
- Improves potency
For Female Health
- As a means of adjuvant therapy for inflammatory diseases of the genital organs
- It is used in cosmetology as a means of improving collagen synthesis and increasing skin elasticity
Sport
- Increases the production of blood cells (red blood cells)
- Improves the saturation of cells with oxygen) and protects them from damage
- Reduces the neural (subjective) feeling of fatigue from physical exertion and is used in athletes (rowing, cycling), which reduces the time required for athletes to distance themselves.
Depression
- Reduces side effects when taking tricyclic antidepressants
- Increases the level of serotonin in the brain, which reduces the manifestations of depression.
- Indications & Contrindications
Indications
- Asthenia
- Neurasthenia
- Vascular dystonia
- Hypotension
- Overwork
- Recovery period
Contrindications- Pregnancy and lactation
- Excitation (activation)
- Hypertensive crisis
- Feverish states
- Autoimmune diseases
Packaging & Availability
- Packaging Information
- Ingredients are packed into double bags to avoid moisture condensation. During shipment bags are stored into double-double carton boxes.
- 10 kg/carton box
Storage & Handling
- Storage and Shelf Life
Stored in a well-closed container in cool and dry place, keep away from direct strong light and heat. Not less than 2 years when properly stored (see COA).