Knowde Enhanced TDS
Identification & Functionality
- Chemical Name
- INCI Name
- Ingredient Name
- Ingredient Origin
- Protein Type
- Cosmetic Ingredients Functions
- Pharma & Nutraceuticals Functions
- CAS No.
- 56-89-3
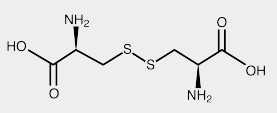
- Molecular formula
- C₆H₁₂N₂O₄S₂
- Ingredients
- L-cystine
- Food Additive Number
- E 920, INS 921
- EC No.
- 200-296-3
- Product Families
- Chemical Structure
Features & Benefits
- Benefit Claims
- Benefit Claims (Health)
Applications & Uses
- Applications
- Application Format
- Hair Care Applications
- Applications
- Excipients and Auxiliaries
- Personal Care
- Pharma
- Application Details
- Excipient for API synthesis, e.g. expectorants
- Essential ingredient in cell culture media
Properties
- Insoluble in
- Alcohol
- Typical Properties
- Composition
- Specifications
| Value | Units | Test Method / Conditions | |
| Molecular Weight | 240.3 | — | — |
| pH (in 0.04 g/l H₂O) | 5 - 6.5 | — | Specific method |
| Solubility in Water (at 20°C) | 0.122 | g/l | EU-GL.A.6 |
| Value | Units | Test Method / Conditions | |
| Active Content | min. (98.5 -101.0) | % | — |
| Value | Units | Test Method / Conditions | |
| Ammonium | max. 0.02 | % | PH. EUR. (2.2.56) |
| Appearance of Solution | Colorless clear liquid | — | PH. EUR. (2.2.1) |
| Arsenic Content | max. 1 | ppm | PH. EUR. (2.4.2/A) |
| Assay | 98.5 - 101.0 | % | PH. EUR. (2.2.20) |
| Chloride Content | max. 200 | ppm | PH. EUR. |
| Heavy Metals | max. 10 | ppm | PH. EUR. (2.4.8/D) |
| Iron Content | max. 10 | ppm | PH. EUR. (2.4.9) |
| Lead Content | max. 5 | ppm | PH. EUR. (2.4.8/D) |
| Loss on Drying | max. 0.2 | % | PH. EUR. (2.2.32) |
| Ninhydrin Positie Substances (each) | max. 0.2 | % | PH. EUR. (2.2.56) |
| Residue on Ignition | max. 0.1 | % | PH. EUR. (2.4.14) |
| Specific Rotation ([α]/D20) | -224 to -218 | Degree | PH. EUR. (2.2.7) |
| Sulfate Content | max. 300 | ppm | PH. EUR. (2.4.13) |
| Total Ninhydrin-Positive Substances | max. 0.5 | % | PH. EUR. (2.2.56) |
Regulatory & Compliance
- Certifications & Compliance
- Grade
- Compliance
- Complies with current Eur.Ph. and FCC specifications
- Non-GMO microorganisms
- BSE/TSE-free
- Natural according Regulation 1334/2008/EC
Packaging & Availability
- Country Availability
- Regional Availability
- Packaging Information
Units of 25 kg.
Storage & Handling
- Shelf Life
- 36 Months
- Storage Information
FERMOPURE® L-CYSTINE PHARMA has a shelf life of at least 36 months when stored in unbroken original packaging in dry storage areas.