Knowde Enhanced TDS
Identification & Functionality
- Active Component
- Ingredient Name
- Pharma & Nutraceuticals Functions
- Ingredients
- Curcumin Extract
- Product Families
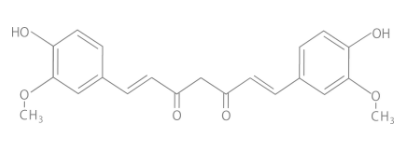
- Chemical Structure
Features & Benefits
- Food Ingredients Features
- Turmeric & Curcumin
What is turmeric?
Turmeric is a perennial plant of the ginger family and is widely spread throughout South and Southeast Asian countries. It is commonly used as a spice, flavoring agent, food preservative, and coloring agent, and is known as a key ingredient of curry powder.
What is curcumin?
Curcumin is a polyphenol contained in the rhizome of turmeric and is considered to be a main active ingredient of turmeric. It has a bright yellow color and is used as a natural food dye.
It is also well known for its effect of anti-inflammatory and anti-oxidant amongst medical and health science laboratories all over the world. A great number of researches are being conducted by a variety of medical and science institutes.
Applications & Uses
- Markets
- Applications
- Food & Nutrition Applications
- Main evidence for THERACURMIN®
- Anti-oxidant &
- Anti-inflammatory Effect
- Osteoarthritis Effect
- Muscle fatigue Effect
- Dementia Effect
- Liver function Effect
- Cardiovascular Effect
- COPD Effect
- Cancer Treatment
- Line Up
A highly absorbent curcumin preparation used as a raw material of functional food.
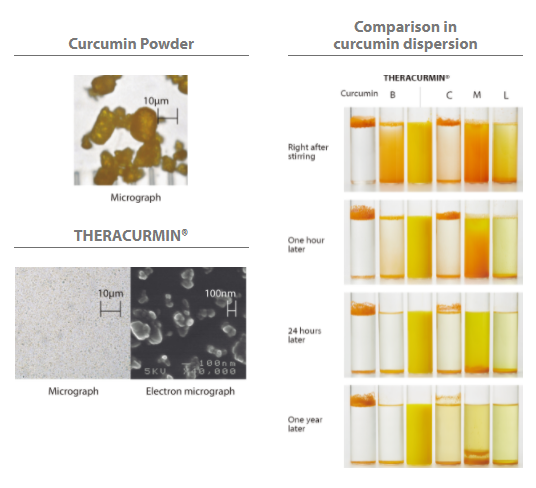
By crumbling the particles of curcumin and maintaining their particle sizes stably, the particles are absorbed by approximately 27-fold. As it also has water dispersion capability and heat and light resistance characteristics, it is applicable to any forms of products as shown below.- Hard capsule
- Tablet
- Granules
- Beverage, Liquid
- Gummy, chewable, gum
- Jelly
- Ice lolly, popsicle, sherbet
- Noodles
Properties
Technical Details & Test Data
- THERACURMIN® Possesses High Bioavailability
Area Under the Curve(AUC)0-6h values of THERACURMIN® were 27-fold higher than those of curcumin powder.
AUC0-24 h was found to be 11.0- and 5- fold higher with TEHRACURMIN® than Company A and Company B, respectively.Study Protocol
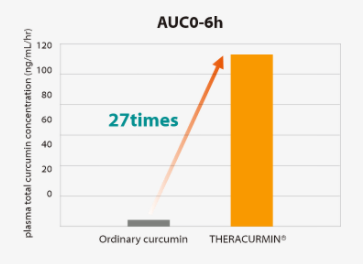
Study design:
Two arms, randomized parallel-group controlled trialSubjects:
14 healthy subjects (male/female=8/6, age: 44.1±8.5 years old)Intake:
-THERACURMIN®:30mg -Ordinary curcumin: 30mgArea Under the Curve(AUC)0-6h values of THERACURMIN® were 27-fold higher than those of curcumin powder.
Study Protocol
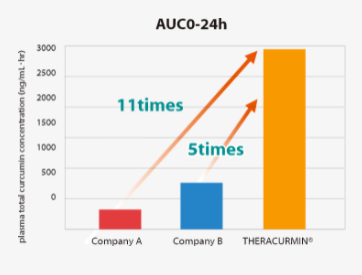
Study design:
Three arms, randomized double-blind 3-way crossover trialSubjects:
9 healthy subjects (male/female=5/4, age: 24-32)Intake:
-THERACURMIN®:180mg
-Company A: 260mg
-Company B: 150mgAUC0-24 h was found to be 11.0- and 5- fold higher with TEHRACURMIN® than Company A and Company B, respectively.
- Product Highlights

- Safety and toxicity data of THERACURMIN®
Toxicity data Test Ames test Chromosome aberration test Micronucleus assay test Acute toxicity study
(Rats)Subacute toxicity study
(Rats)Subchronic toxicity study
(Rats)Test detail Salmonella spp.
(4 strains)
E.ColiIn vitro shromosome
aberration test in
Chinese hamster lung cellsMale sprague dewley rats
bone merrow micronucles assayDose: 1250,2500,5000mg/kg
(As curcumin 375,750,1500mg/kg)
OnceDose: 0,625,1250,2500,5000mg/kg
(As curcumin 0,187.5,375,750,1500mg/kg)
Period: 2 weeksDose: 0,1250,2500,5000mg/kg
(As curcumin 0,375,750,1500mg/kg)
Period: 13 weeksResult Negative Negative Negative No toxicological findings observed by oral administration No toxicological findings observed by oral administration No toxicological findings observed by oral administration
Clinical study in healthy volunteers Test 4-week clinical study in healthy volunteers at excessive dosage 12-week clinical study in healthy volunteers at standard dosage Test detail Tested dosage: 900mg/q.d./p.o. (as curcumin, 5x of standard dosage)
Period: 4weeksTested dosage: 180mg/q.d./p.o. (as curcumin standard dosage)
Period; 12weeks.Result THERACURMIN® was proved safe in this study. THERACURMIN® was proved safe in this study. - q.d.: Once a day
- p.o.: Oral intake
- ADI: Acceptable Daily Intake
- Standard dosage: 180mg/day (as curcumin)= ADI: 3mg/kg x 60kg (average adult body weight)
Previous clinical study Study title Memory and Brain Amyloid and Tau Effects of a Bioavailable Form of Curcumin in Non-Demented Adults: A Double-Blind, Placebo-Controlled 18-Month Trial. 12-week clinical study in healthy volunteers at standard dosage Test detail Subject: Non-Demented Adults
THERACURMIN® dose: 180mg/day
Period: 18 monthsSubject: Patients with stage Ⅰ-ⅡCOPD
THERACURMIN® dose: 180mg/day
Period: 6 monthsResult No serious adverse effects were obsevered in this study No serious adverse effects were obsevered in this study

