Knowde Enhanced TDS
Identification & Functionality
- Carrier
- INCI Name
- Ingredient Origin
- Cosmetic Ingredients Functions
- CAS No.
- 34769-44-3, 39012-86-7
- EC No.
- 254-248-1, 252-204-6
- Technologies
- Product Families
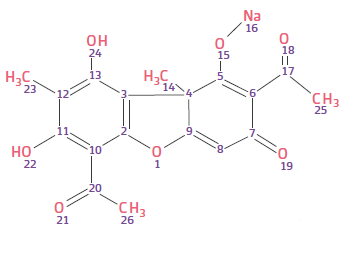
- Structure of Sodium Usnate
Features & Benefits
- Labeling Claims
- Product Overview
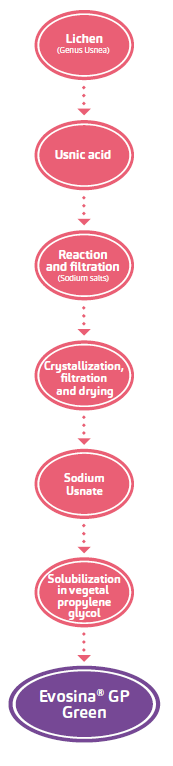
Origin and structure
- Usnic acid is a lichenic acid present in a wide number of species and varieties of lichens including genus Usnea, Cladonia,Cetraria and Parmelia.
- Several lichens have been used in the past in traditional medicine for making infusions and extracts. In particular, in ancient China and Arabia the use of Usnea lichen extracts for the treatment of cutaneous infections is documented.
- Usnea barbata, vulgarly called “olds man’s beard”, for its wide diff usion and being a not protected lichen species, represents the primary source for the industrial production of usnic acid.
- Usnic acid possesses strong antibacterial properties probably associated to its peculiar chemical structure.
- However, its poor solubility profi le in most of the solvents regularly used by the cosmetic industry, narrows its use in cosmetic products.
- The salification of usnic acid with sodium salts lead to an improved solubility, maintaining the same activity profile.
- Th e resulting active ingredient is denominated Evosina® (Sodium Usnate).
Naturalness
- Evosina GP Green is a 100% natural origin ingredient.
- Sodium Usnate and propylene glycol are raw materials of vegetable origin, they are respectively obtained from lichen and glycerin from rapeseed and sunfl ower oils.
- Th e starting materials and the manufacturing process guarantee a 100% Natural Origin Index according to ISO 16128.
- Cosmetic Properties
Evosina® (Sodium Usnate) possesses marked antibacterial properties mainly addressed
towards gram+ strains and some fungi species (eg Malassezia furfur), resulting perfectly suitable for deodorant applications, acne prone skin, anti-dandruff cosmetic products.
Applications & Uses
- Markets
- Applications
- AP/Deo Applications
- Hair Care Applications
- Personal Hygiene Applications
- Skin Care Applications
- Treatment Product Applications
- Use Level
- 0.1 - 1%
- Note for formulator
Evosina® GP Green can be added in emulsion during the cooling process under stirring. It can be also used in most of detergent systems, incorporating the specialty at the end of the process before making pH adjustments.
- Applications
- Evosina® GP Green thanks to its marked and specifi c antimicrobial profi le can be considered an effective natural alternative for deodorant, acne prone skin and anti-dandruff cosmetic products.
- A range of suggested formulations incorporating Evosina® GP Green includes: deodorant sprayable solution, intimate hygiene detergent formulation, sprayable deodorant cream, anti-acne cream, anti-dandruff shampoo, foot deodorant creams and powders.
- Evosina® GP Green is listed by Cosmos as natural derived ingredient and therefore can be used in certifi ed natural cosmetic products.
- Deo sprays & roll-ons, face cleansers, emulsions for acne prone skin treatments, anti-dandruff shampoo. Oral care products.
- Skin Care
- Hair Care
- Make Up
- Toiletries
Properties
- Physical Form
- Odor
- Characteristic
- Appearance
- Liquid
- Typical Properties
- Physico-Chemical Properties
- Microbiological Values
- Composition
- Origin-Properties
Usnic acid sodium salt, natural derived from lichens (gen. Usnea), in natural origin propylene glycol. High antimicrobial activity against Gram+ bacterial strains (e.g. Corynebacteria, Staph. epidermidis, Propionibacterium acnes).
| Value | Units | Test Method / Conditions | |
| Heavy Metals | max. 20 | ppm | — |
| Preservatives | Absent | — | — |
| Value | Units | Test Method / Conditions | |
| Active Fraction | 1.8 - 2.2 | % | NCQ 1694 |
| Color | 0 - 12 | Gardner | NCQ 2194 |
| Density | 0.991 - 1.091 | — | NCQ 0494 |
| Refractive Index | 1.428 - 1.448 | — | NCQ 0594 |
| Value | Units | Test Method / Conditions | |
| Pathogens | Absent | — | — |
| Total Microbial Count | max. 100 | cfu/g | EQC0189 |
| Yeast and Mould Count | max. 10 | cfu/g | EQC0019 |
| Value | Units | Test Method / Conditions | |
| Propylene Glycol | 98 | % | — |
| Sodium Usnate | 2 | % | — |
Regulatory & Compliance
- Certifications & Compliance
- Chemical Inventories
- Legislative Compliance
EU: approved
USA: approved
China: approved, product listed in IECIC 2015
Technical Details & Test Data
- Efficacy studies
Evosina® effi cacy has been tested on diff erent Gram+ strains. The in-vitro average MIC of Evosina® (Sodium Usnate) turned out 5 ppm, lower than the value found for Usnic acid (10 ppm).
Gram +Strain tested MIC
Concentration
(in ppm)20 10 5 2 » Staphilococcus aureus - - + + » Staphilococcus epidermidis - - - - » K. Rhizophila - - - - » S. Pyogenes - - - + » Bacillus cereus - - - - » B. Spizizenii - - - - » Enterococcus hirae - - + + MIC values of Evosina® (Sodium Usnate) vs Gram+ strains
Evosina® (Sodium Usnate) turned out very eff ective towards Propionibacterium acnes as well.
Strain tested MIC » Propionibacterium acnes 0.01% MIC of Evosina® (Sodium Usnate) vs Propionibacterium acnes
Evosina® GP Green also showed a good antimicrobial power towards Malassezia furfur.
Strain tested MIC » Malassezia furfur 0.1% MIC of Evosina® GP Green vs Malassezia furfur (P. Ovale)
Process
Packaging & Availability
- Packaging Information
- 5 - 25 Kg Bags
Storage & Handling
- Shelf Life
- 1 year
- Storage Information
Retest Date: 1 year from the date indicated on the certificate of analysis.
Stored in sealed packaging and avoid contact with light and heat sources.
For further information on handling see the product safety data sheet.

