Knowde Enhanced TDS
Identification & Functionality
- Ingredient Name
- Food Ingredients Functions
- Pharma & Nutraceuticals Functions
- Ingredients
- Bifidobacterium Lactis, Lactobacillus Acidophilus, Lactobacillus Salivarius, Lactobacillus Casei, Bifidobacterium Bifidum, Lactococcus lactis, Lactobacillus brevis
Features & Benefits
- Food Ingredients Features
- Product Highlights
Today, a record number of patients worldwide suffer from metabolic disorders, including obesity, non-alcoholic fatty liver disease (NAFLD), type 2 diabetes mellitus (T2DM) and cardiometabolic disease (CMD)1 . As poor diets, lack of exercise, and other stressors continue to negatively impact millions of people around the globe, we must look for new ways to improve metabolic health, delay disease progression, and foster a better quality of life where possible.
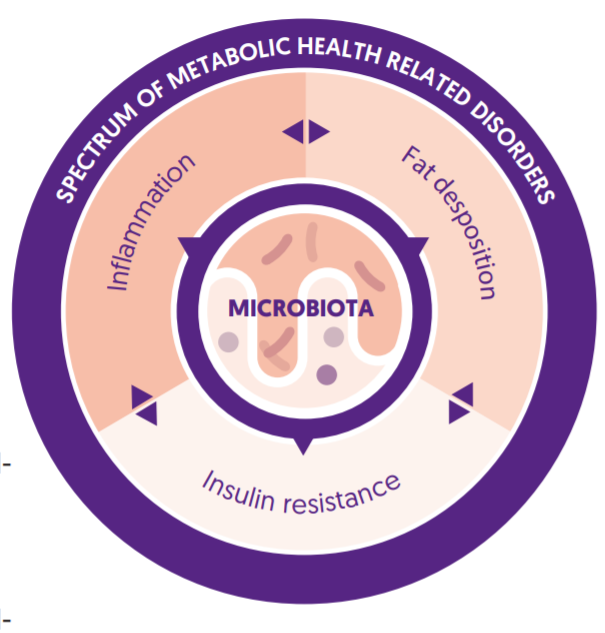
FIGURE 1: Crosstalk between gut microbiota and host’s system in terms of inflammation and metabolism. The gut microbiota, through a range of molecular interactions, contribute to host insulin resistance, systemic low-grade inflammation, and fat deposition and therefore, indirectly participate in the onset and progress of (metabolic) diseases.
Insulin resistance and systemic low-grade inflammation seem to be at the core of metabolic disorders2–4. Recent research has indicated that the gut microbiota plays an important role in managing metabolic health5,6. Disturbance of gut microbiota by a typical western lifestyle leads to changes in serum lipopolysaccharides (LPS), short-chain fatty acids (SCFAs) and bile acid, resulting in systemic low-grade inflammation and insulin resistance5,7,8. [see figure 1] Given the role the microbiota on metabolic disorders, targeted probiotic formulations may be clinically relevant for optimizing metabolic health, influencing insulin resistance and systemic low-grade inflammation associated in earlyand late-stage metabolic disorder, specifically T2DM. Recent literature has supported the efficacy of probiotics for improving a range of metabolic markers, including HOMA-IR, a measure of insulin resistance, and serum LPS, a measure of gut permeability and a trigger of inflammatory responses9–13.
- Strain Selection
Ecologic® BARRIER is a multispecies probiotic formulation consisting of 9 specifically selected probiotic strains. Probiotics strains can exert health effects at different levels in the gut [figure 2 3-levels of action]. These strains were selected based on their ability to strengthen the intestinal barrier function (level 2) and reduce low-grade inflammation (level 3)14, making it a suitable choice for research in insulin resistance and metabolic health.
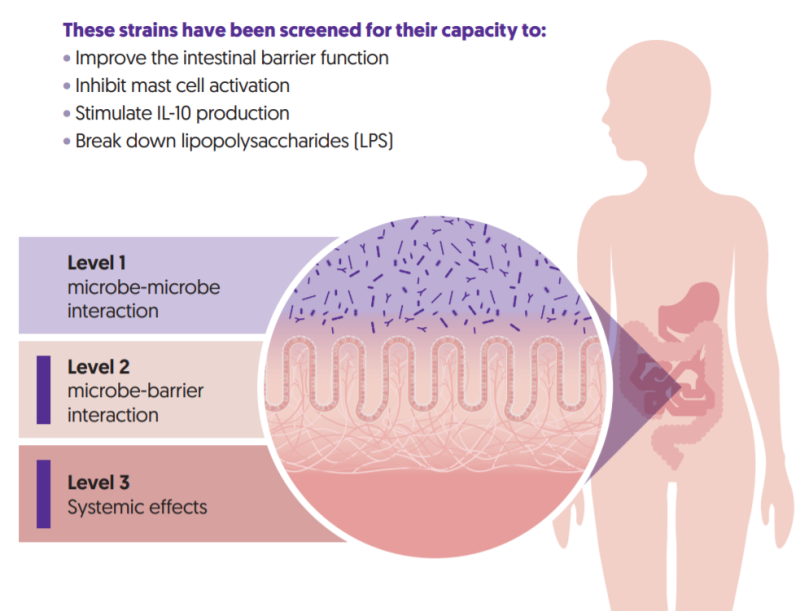
FIGURE 2: Probiotic strains can be active on three levels in the gut. The strains in Ecologic® BARRIER have been proven active at level 2 and 3
Applications & Uses
- Markets
- Food & Nutrition Applications
Properties
Regulatory & Compliance
- Certifications & Compliance
Technical Details & Test Data
- Clinical Evidence
Ecologic® BARRIER has been tested in a double-blind, placebo-controlled, randomized study, performed by the Warwick University, UK and King Saud University, Saudi Arabia9,10. Ninety-six adult T2DM patients (treatment-naïve and without co-morbidities) were randomized to receive 2 grams of Ecologic® BARRIER twice daily (1.0×1010 cfu/day) or placebo for 6 months. In the probiotic group Ecologic® BARRIER significantly reduced HOMA-IR levels after 3 months and 6 months, which did not occur in the placebo group (see figure 3). In line with this a significant decrease in fasting glucose and fasting insulin was observed in the probiotic group. In addition, Ecologic® BARRIER intake reduced circulating endotoxin levels (LPS), a trigger of inflammation and a marker for gut barrier function, and improved inflammation markers such as CRP, TNF-α, IL-6
The positive effect of Ecologic® BARRIER on the gut barrier function was also observed randomized, double-blind, placebo-controlled pilot study performed by researchers from the Medical University of Graz, Austria13. In this study, twenty-six treatmentexperienced obese T2DM patients were randomized to receive 6 grams of Ecologic® BARRIER (1.5x1010 cfu/day) and a prebiotic or a placebo daily for 6 months. After 3 months patients in the placebo group showed a degraded gut permeability (increase in serum zonuline) which was not observed in the Ecologic® BARRIER plus prebiotic group. Another double-blind, placebo-controlled randomized study performed by the University of Medical Sciences in Poznan, Poland studied the effects of Ecologic® BARRIER on the metabolic health of obese postmenopausal women11. Eighty-one obese postmenopausal women were randomly assigned to receive placebo, a low dose of Ecologic® BARRIER (LD) (2.5x109 cfu/day), or a high dose of Ecologic® BARRIER (HD) (1x1010 cfu/day) divided in two equal doses for 12 weeks. Both LD and HD Ecologic® BARRIER intake resulted in significantly reduced HOMA-IR levels compared to baseline, which was not observed in the placebo group. A dose-response effect was observed as a significant larger reduction of HOMA-IR occurred in the HD group (see figure 4). Moreover, Ecologic® BARRIER improved circulating endotoxin (LPS) levels. A second publication of the same clinical trial showed that inflammation makers such as TNF-α, IL-6 and functional and biochemical markers of vascular dysfunction such as blood pressure improved as well12.
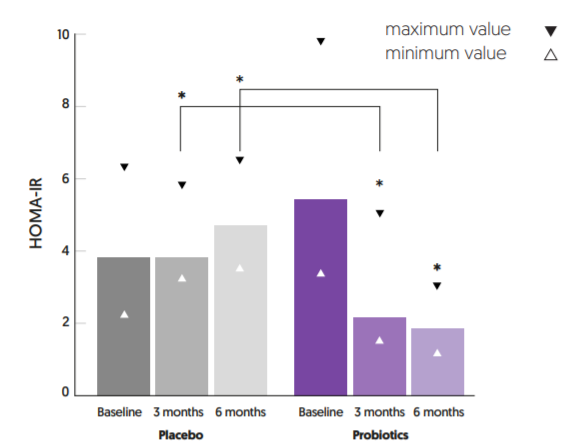
FIGURE 3: HOMA-IR levels (Median (range)) before and after 3 months and 6 months supplementation with Ecologic® BARRIER. *Significant decrease, p<0.05
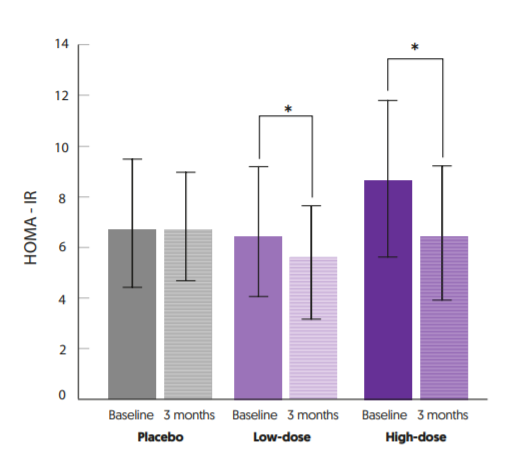
FIGURE 4: HOMA-IR levels (Mean ± SD) before and after 12 weeks of low or high dose supplementation with Ecologic® BARRIER. *Significant decrease, p<0.05

