Knowde Enhanced TDS
Identification & Functionality
- Cosmetic Ingredients Functions
- Technologies
- Product Families
Features & Benefits
- Benefit Claims
- Labeling Claims
- Benefits
- Benefits In Anti-Aging Formulations: Retinaldehyde is one step closer to efficacy than Retinol. Retinol and Retinaldehyde are converted to retinoic acid (tretinoin) naturally in the skin in order to provide efficacy.
- Retinol requires two oxidation steps in the skin - first by converting the retinol to retinaldehyde and then to retinoic acid.
- Retinaldehyde only requires the one oxidation step to produce retinoic acid.
- Retinaldehyde is a cosmetic ingredient (not an Rx or OTC active) so it can be used in a formulation without complicated regulatory support.
- MICROSPONGE® 520RA Retinaldehyde Benefits in Your Formulation:
- Sustained Release (Time Release) of Retinaldehyde.
- BHA-Free - California Proposition 65 compliant.
- Benefits In Anti-Aging Formulations: Retinaldehyde is one step closer to efficacy than Retinol. Retinol and Retinaldehyde are converted to retinoic acid (tretinoin) naturally in the skin in order to provide efficacy.
Applications & Uses
- Markets
- Applications
- Skin Care Applications
- Formulation Guidelines
While Microsponge® 520RA Retinaldehyde can help improve the stability of retinaldehyde in your product, it still requires care to provide the optimum benefit. Retinaldehyde should always be handled under yellow light to prevent isomerization and exposure to oxygen should be minimized by sparging the formulation with nitrogen and adding the 520RA during the cool down phase of making an emulsion. Once the original package has been opened, use all of the 520RA or reseal the container.
Properties
- Appearance
- Yellow, free flowing powder
- Typical Properties
| Value | Units | Test Method / Conditions | |
| Retinaldehyde Concentration | 20.0 +/- 2 | % | — |
Technical Details & Test Data
- Sustained Release Of Retinaldehyde – Franz Diffusion Study
The ability of the Microsponge® delivery system to provide sustained release retinaldehyde is shown in the following test results. Cosmetic creams were made that contained 0.10% retinaldehyde – one that contained free retinaldehyde (added to the oil phase of the emulsion) and one that contained the same amount of retinaldehyde that was contained in the Microsponge® delivery system. Both creams were evaluated via Franz cell diffusion testing that determine the release rate of the Retinaldehyde through a membrane that simulates human skin. The results of the testing is shown below.
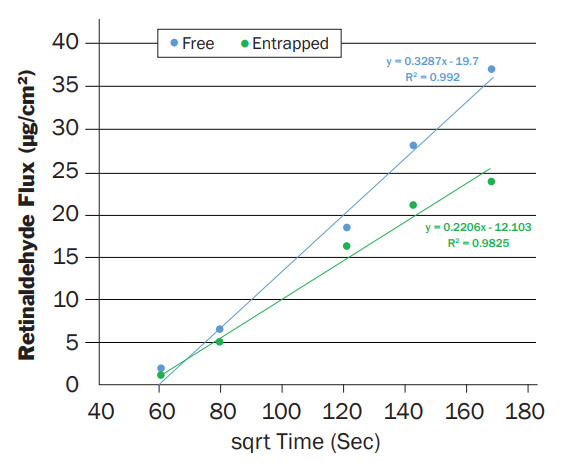
The results of the testing indicate that entrapping the retinaldehyde in the Microsponge® delivery system results in a slower release of the active in comparison to the “Free” retinaldehyde. Similar testing with other irritating actives has shown that sustained release can reduce irritation and result in improved consumer acceptance/utilization of antiaging products that leads to improved efficacy.
- Retinaldehyde Stability - Ingredient And In Formulation
Retinoids are known to be sensitive to oxidation and a resultant loss of efficacy during storage as both an ingredient and as finished products. The following studies were conducted to demonstrate the stability of Microsponge® 520RA as an ingredient and in formulation.
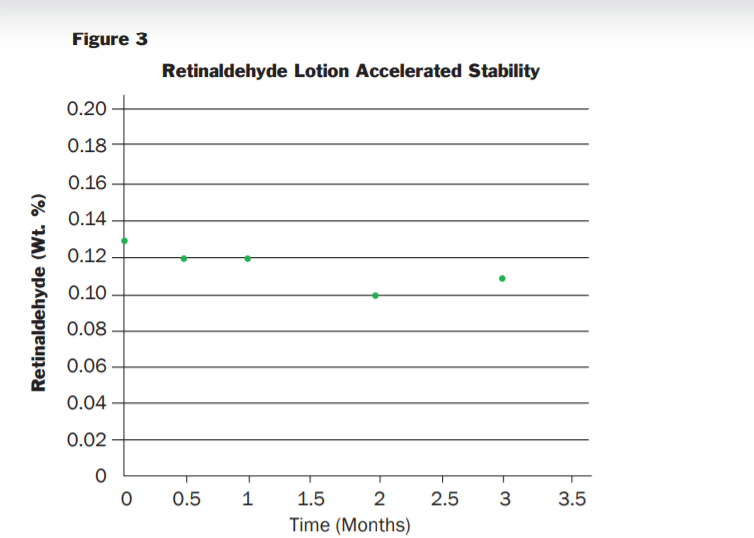
Microsponge® 520RA ingredient was placed on stability under ambient conditions in a glass container purged with inert gas. Samples were withdrawn from the container at timed intervals and analyzed for Retinaldeyde content via HPLC (the container was maintained under inert gas during the sampling). The results of the testing are provided in Figure 2 The results indicate that entrapment remained in specification for the duration of the study (44 Weeks) even though the container was opened multiple times to collect the assay samples. The Microsponge® 520RA also showed excellent stability in a cosmetic formulation. Figure 3 shows the stability testing of a 0.10% retinaldeyde cream tested in 30 mL foil laminate tubes for 12 weeks at 40°C
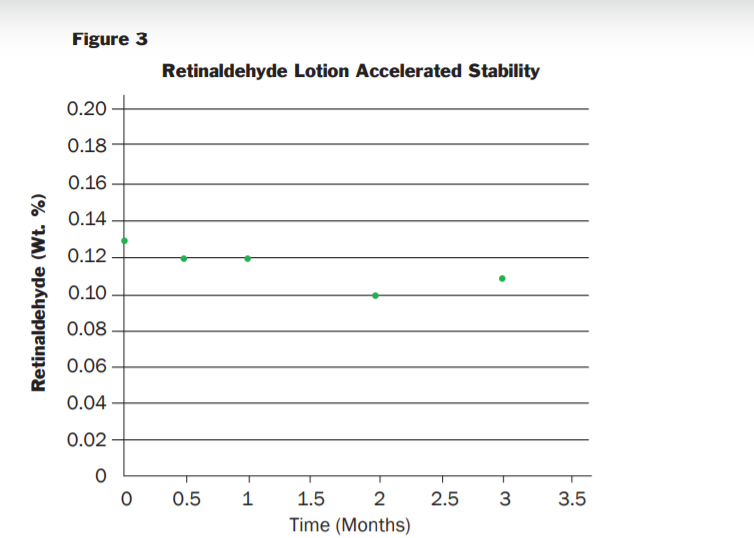 The results show that the formulation maintains an in-specification retinaldeyde concentration for the duration of the study and supports a 2-year shelf life. The ingredient list for the formulation is provided below and more details on the production of this cream can be obtained by contacting your HBS representative. Example Microsponge® 520RA formulation (0.10% Retinaldehyde) ingredient list: Water, Caprylic/Capric Triglyceride, Glycerin, Ceteraryl Alcohol, C10-30 Cholesterol/Lanosterol Esters, Dimethicon, Cetyl Ricinoleate, Cetyl Alcohol, Polysorbate 60, Phenoxyethanol, Stearic Acid, Cyclopentasiloxane, PEG-10 Soy Sterol, Magnesium Aluminum Silicate, Methyl Methyacrylate/Glycol Dimethacryalte Copolymer, Cyclohexasiloxane, Chlorphenesin, Disodium EDTA, Xanthan Gum, Retinaldehyde, BHT, Tocopheryl Acetate, Ethylhexylglycerin, Bisabolol, Sodium Hydroxide, Ascorbyl Palmitate.
The results show that the formulation maintains an in-specification retinaldeyde concentration for the duration of the study and supports a 2-year shelf life. The ingredient list for the formulation is provided below and more details on the production of this cream can be obtained by contacting your HBS representative. Example Microsponge® 520RA formulation (0.10% Retinaldehyde) ingredient list: Water, Caprylic/Capric Triglyceride, Glycerin, Ceteraryl Alcohol, C10-30 Cholesterol/Lanosterol Esters, Dimethicon, Cetyl Ricinoleate, Cetyl Alcohol, Polysorbate 60, Phenoxyethanol, Stearic Acid, Cyclopentasiloxane, PEG-10 Soy Sterol, Magnesium Aluminum Silicate, Methyl Methyacrylate/Glycol Dimethacryalte Copolymer, Cyclohexasiloxane, Chlorphenesin, Disodium EDTA, Xanthan Gum, Retinaldehyde, BHT, Tocopheryl Acetate, Ethylhexylglycerin, Bisabolol, Sodium Hydroxide, Ascorbyl Palmitate.

