Knowde Enhanced TDS
Identification & Functionality
- Ingredient Name
- Ingredient Origin
- Food Ingredients Functions
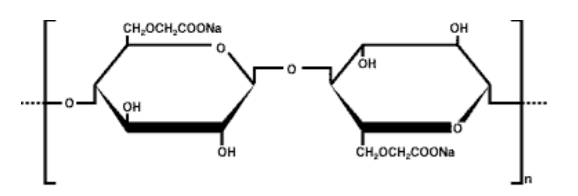
- Formula
- C6H9O4OCH2COONa
- Other Names
- CMC, Cellulose Gum, NaCMC
- Food Additive Number
- E 466, INS 466
- Technologies
- Product Families
- Origin and Production Method
Product coming from cellulose. Cellulose ether product of the reaction between cellulose, sodium hydroxide and monochloroacetic acid with alcohol as a reaction medium at high temperatures; Depending on the final technical characteristics, sodium percarbonate can be used as a viscosity regulator and /or sodium hypochlorite as a bleaching agent, especially when the final destination of the product is the pharmaceutical industry or personal care industry (toothpaste). Any types of additive or reaction coadjuvants are involved in its process.
- Chemical Formula
Features & Benefits
- Labeling Claims
Applications & Uses
- Markets
- Applications
- Food & Nutrition Applications
- Applications
This kind of CMC is used as a thickener, stabilizer, and water withholding agent in the food industry and Pharmaceutical industry . This product is not for direct consumption
- Preparation Before Use
The doses to be used and their form of preparation will depend on each type of application; this material does not require any special handling prior to its use.
- Use
- Expected use
- Gelycel is used as a thickener, stabilizer and water retention agent in the following food industries: baking, powdered juices, ice cream, meat, sauces, dairy products, mayonnaise, pickles, confectionery, pastry, etc. It is not a product for direct consumption. The CMC can be used as a food additive without any restriction in any type of food application (quantum satis). So we suggest that each client evaluated the maximum dose suggested or required to obtain the characteristics of their products, because we have a reference of laxative effects with the product at very high dosage levels. The product can be used in Kosher and Halal diets without restriction, it can also be used in vegan diets since it is made from plant material and not raw materials of animal origin are used. We don’t have knowledge or references that this product can not be consumed by children, elderly persons or women in the gestation stage.
- Use Not Foreseen
- The CMC is a versatile product, it can be used in many types of industries such as oil, textiles, construction, paints, among others, which makes it difficult to restrict its use, which is why Amtex defines different production lines and assigns the letter F for those products that due to their purity and substitution are recommended to be used by the pharmaceutical or food industry. Where these two factors are not restrictive is used interchangeably, bearing in mind that it should not be used as a lubricant for equipment or absorbent material in the food industry.
- Expiration
- Amtex S.A. defines as expiration criterion for its products a period of 2 years from the date of manufacture, this time is only provided as a reference for our clients to make a preferential consumption of their inventories and thus avoid that the products lose any of its properties. However, due to the nature of the product and as a result of a natural process, the viscosity may decrease over time, still outside the specification range, but the product remains safe for use, so we recommend that the material be re-evaluated after a long period of storage. and if necessary, make a small correction in the dosage levels to maintain an optimal behavior in the application. Obviously, this is only possible when the product has received proper handling in storage.
Properties
- Odor
- Odorless
- Flavor
- Flavorless
- Physico-Chemical Properties
- Microbiological Values
- Heavy Metals
| Value | Units | Test Method / Conditions | |
| Moisture | max. 8 | % | ASTM-D1439 |
| pH Value (Solution 1 wt%, 25°C) | 6.5 – 8.5 | - | Amtex method |
| Purity DB (w/w) | min. 99.5 | % | ASTM-D1439 |
| DS (Degree Of Dubstitution) | 0.65 – 0.90 | - | ASTM-D1439 |
| Viscosity Brookfield LVF (DB , 1 wt%,25°C, Sp 3 , 30 rpm) | 3000 - 4000 | cps | ASTM-D1439 |
| Retention M-40 (w/w) | max. 8 | % | Amtex method |
| Value | Units | Test Method / Conditions | |
| S. Aureus | Absent/g | - | - |
| Fecal Coliforms | Absent/g | - | - |
| Salmonella SPP | Absent/25 g | - | - |
| Total Aerobic Plate Count | max. 1000 | CFU/g | - |
| Moulds and Yeast | max. 1000 | - | - |
| Value | Units | Test Method / Conditions | |
| Lead | max. 2 | ppm | AOAC |
| Arsenic | max. 2 | ppm | AOAC |
| Cadmium | max. 0.5 | ppm | AOAC |
| Copper | max. 5 | ppm | AOAC |
| Mercury | max. 1 | ppm | AOAC |
| Sum of Heavy Metals | max. 20 | ppm | AOAC |
Regulatory & Compliance
- Certifications & Compliance
- Regulatory Status
The product complies with the regulations suggested by JECFA-WHO (E466) and 21 CFR 182.1745 as an additive considered as GRAS (Generally Recognized As Safe) by the United States FDA. It complies with the regulations of the European Community EU 231/2012 and the Codex COEI-1- CMC: 2009 It also complies with resolutions 1506 of 2011 on labeling and resolution 2606 of 2009 for food additives, of the Ministry of Social Protection of Colombia
- Destination Market
- The CMC is distributed nationally and internationally complying with all the requirements demanded by the FDA and European Community (EC) , its labeling complies with the requirements of Resolution 1506 of 2011 of the Ministry of Social Protection of Colombia or the regulation that modifies, adds or replaces it and the resolution 2606 of 2009 of the Ministry of Social Protection of Colombia or the regulation that modifies, adds or replaces of technical regulations for food additives. This product also meets the requirements established in the Codex Stan 192-1995 GSFA Rev 2017.
Technical Details & Test Data
- Technical Details
Nutrition facts (per 100 gr)
Item Description Calories+ 0 kcal Total fat 0,00 g Cholesterol 0,00 g Sodium 9,50 g aprox Calcium 5,00 mg max Iron 10 ppm max Carbohydrates 0,0 g Dietary fiber++ 85 g max. Soluble Fiber 85 g max. Insoluble Fiber 0,1 g max. Proteins 0,0 g Vitamins 0,0 g Complementary parameters
Item Description Allergens Absents GMO (Promoter CaMV35S - Promoter FMV 34S - Terminator NOS) Absents Pesticides Absents Volatile organic impurities Not detectable Microtoxins, aflatoxins, zearelenone. Absents Foreing Materials Absents
Packaging & Availability
- Packaging Type
- Packing and Presentation
The CMC comes in bags of 25 kilos with multi-paper covers and an inside plastic liner. The name of the product, the specification number, date of manufacturing, lot number and other requirements made by the client are printed on the bags. The lot number is composed of 4 to 6 digits, the first digits correspond to the consecutive production number and the last two digists correspond to the current year. Example: 112316, it would be the consecutive number of lot 1123 of the year 2016
Storage & Handling
- Storage and Distribution
CMC must be stored in a cool and dry place, best indoors. The product is a hygroscopic solid which tends to absorb moisture from the air, the bags must be closed at all times. If the content is partially consumed, close it again as tightly as possible. The recommended storage temperature is maximum 40 ° C. It is stored at room temperature in pallets forming one-ton pallets and protected externally with plastic. For transportation and distribution, should be vehicles suitable for transporting of food, ensuring that It are not loaded simultaneously with goods whose characteristics are different or have some degree of incompatibility with our product, such as aromas or essences, dyes, among others. The loading and unloading process must be carried out taking care that the packing material does not suffer damages that expose the product to external contaminations or that exposes it to moisture. Temperature control is not required during transport and distribution.

