Knowde Enhanced TDS
Identification & Functionality
- Chemical Name
- Pharma & Nutraceuticals Functions
- CAS No.
- 10039-26-6
- Technologies
Features & Benefits
- Key Benefits
- Compressibility
- Blending properties
- Storage stability
Applications & Uses
- Markets
- Dosage Form
- Manufacturing Technology
- Applications
- Wet granulation
- Dry granulation
- Blends Premixes /Triturations
- Fermentation processes
Properties
- Appearance
- White crystalline powder
- Soluble in
- Water
- Insoluble in
- Ethanol (96%)
- Specifications
| Value | Units | Test Method / Conditions | |
| Absorbance (Proteins and Light Absorbing Impurities, [A] 1%, 1 cm at 210 to 220 nm) | max. 0.25 | — | — |
| Absorbance (Proteins and Light Absorbing Impurities, [A] 1%, 1 cm at 270 to 300 nm) | max. 0.07 | — | — |
| Absorbance (Proteins and Light Absorbing Impurities, [A] 10%, 1 cm at 400 nm) | max. 0.04 | — | — |
| Acidity or Alkalinity (at ml 0.1 M NaOH) | max. 0.4 | — | — |
| Escherichia Coli | Negative | in 1g | — |
| Heavy Metals | max. 5 | ppm | USP |
| Loss on Drying | max. 0.5 | % | — |
| Particle Size Distribution (max. 100 µm) | min. 90 | % | Air Jet Sieve |
| Particle Size Distribution (max. 32 µm) | 45 - 75 | % | Air Jet Sieve |
| Residue on Ignition / Sulphated Ash | max. 0.1 | % | — |
| Salmonella | Negative | in 10g | — |
| Specific Optical Rotation (at [α] 20D, Anhydrous Substance) | 54.4 - 55.9 | degree | — |
| Total Aerobic Microbial Count | max. 100 | CFU/g | — |
| Total Yeast and Molds Count | max. 50 | CFU/g | — |
| Water Content | 4.5 - 5.5 | % | KF Method |
Regulatory & Compliance
- Certifications & Compliance
- Grade
- Residual Solvents
No class 1, 2, 3 solvents are used during production. (CPMP/ICH/283/95 + CVMP/VICH/502/99)
- Certifications
- EXCIPACT certified (GMP)
- ISO 9001 and ISO 22000 certified
- Following IPEC cGMP
- On-site Batch testing & Batch release
- Certified animal Rennet-free raw materials
Technical Details & Test Data
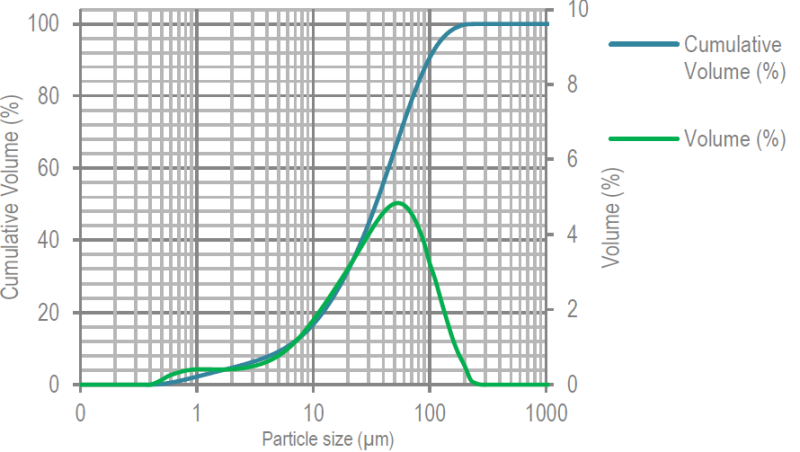
- Particle Size Distribution
PSD
(Air Jet Sieve)Specifications (%) % < 32 µm 45 - 75 % < 100 µm ≥ 90 Due to the presence of fine milled particles, this α lactose monohydrate combines good compaction and blending properties.
PSD (Malvern Laser Diffraction, Indicative Values)
X10 : 6 µm, X50 : 34 µm, X90 : 98 µm
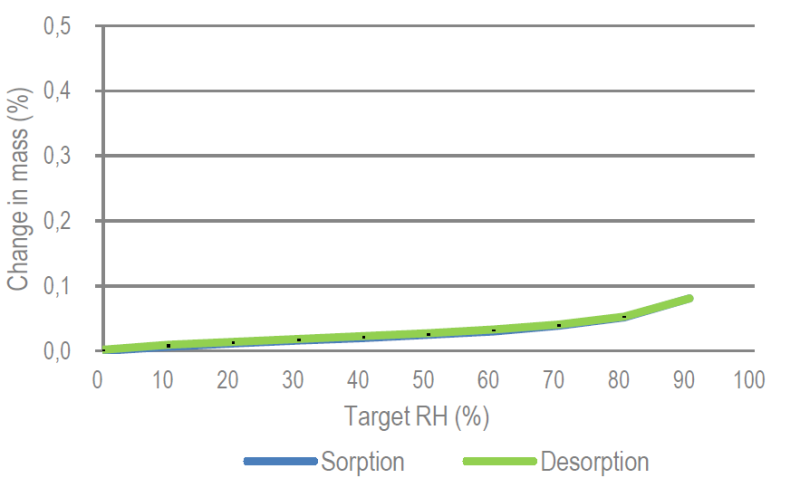
- Moisture Sorption
The hygroscopicity of α lactose monohydrate is very low which enables high stability both in storage as well as in formulations
DVS (Dynamic Vapor Sorption)
- Flowability
- Poured Density : 0.50 g/mL
- Tapped Density : 0.80 g/mL
- Carr’s Index : 37 %
- Hausner Ratio : 1.58
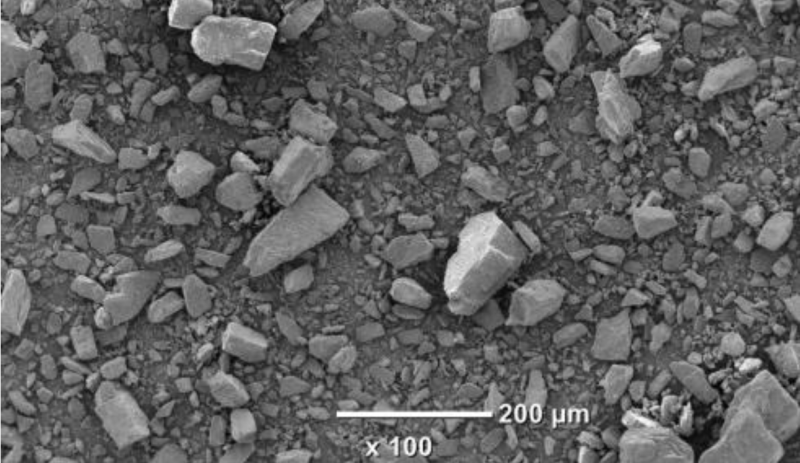
- Scanning Electron Microscopy
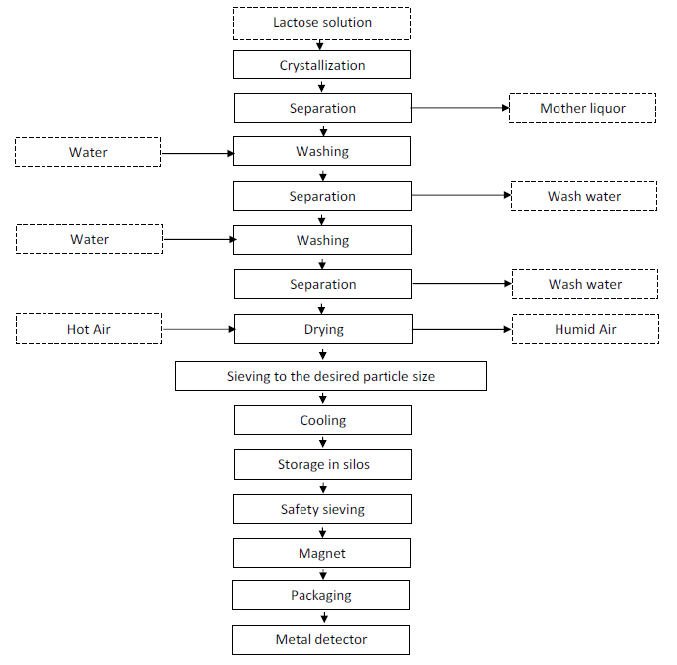
- Process Flow Chart
Packaging & Availability
- Packaging Type
Storage & Handling
- Shelf Life
- 36 Months
- Storage Information
Store in original unopened packaging, in a dry and odor free place.

