Knowde Enhanced TDS
Identification & Functionality
- Food Ingredients Functions
- Pharma & Nutraceuticals Functions
Features & Benefits
- Benefit Claims (Health)
- Labeling Claims
Applications & Uses
- Markets
- Dosage Form
Technical Details & Test Data
- Clinical Data
Nasaler and Allergic Rhinitis:
- Through its unique formulation techniques, bioXtract has managed to create with NASALER a product with significantly higher bioavailability than the individual pure extracts, as proven by our in vivo trials.
- These active principals act on the symptoms of allergic rhinitis, caused by inhaled substances (for example pollen, dust mites, mold spores) to relieve.
- Itching, sneezing and runny eyes and nose - through the properties of natural antihistamine quercetin,
- Nasal congestion, and the underlying inflammation in the nasal membranes, through the broad spectrum anti-inflammatory effects of curcumin (in an improved bioavailable formula).
- At this level of availability, the quercetin and curcumin in NASALER will together have an effect on both the immediate symptoms of allergic rhinitis and the later stage nasal congestion.
Nasaler Is Delivered With a Complete Package of Information, Including Bioxtract Proprietary:
- Pre-clinical efficacy trial evaluating the action of active principles on several models of allergic inflammation in the nasal membrane.
- Pre-clinical acute oral toxicity trial in rats showing no particular effect and classifying the product in the danger 5 category DL50 superior 5,000 mg/kg.
- Phase I clinical trial, assessing the bioavailability of NASALER's curcumin.
- Open-label clinical trial data showing the efficacy of NASALER on the symptoms of allergic rhinitis.
- Clinical Trial Data
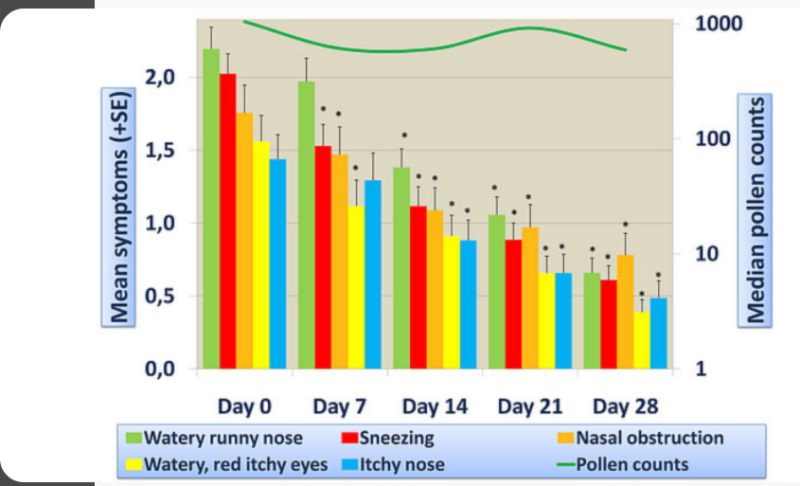
Open-label clinical trial data showing efficacy of NASALER on the symptoms of allergic rhinitis.
- Significanty decreases all symptoms of allergic rhinitis
- Significantly imroves Quality of Life of allergic patients
- Improvement noted wihtin 7 days with 2 capsules a day

