Knowde Enhanced TDS
Identification & Functionality
- Ingredient Name
- Food Ingredients Functions
- Pharma & Nutraceuticals Functions
- Ingredients
- Curcumin
- Food Additive Number
- E 100(i), INS 100, INS 100(i)
Features & Benefits
- Benefit Claims (Health)
- Labeling Claims
- Food Ingredients Features
- Product Background
- Our Co-Grinding Solvent Free (CGSF) technology[ transforms insoluble curcumin in the hydrophobic core of excipient.
- The hydrophilic shell of the excipient is compatible with water and is used to assemblea nano-micelle structure, “hydrophobic core-hydrophilic shell”, to directly increase the solubility of curcumin.
- It can quickly form a gel in vivo and stick into the actionsite, delay the release of curcumin, and further increase the curcumin concentration in the blood to increase bioavailability.
- Product Highlights
- Cost-effective, only 3 cents per day to meet health needs
- 60% curcumin potency
- High water dispersibility
- Clinically proven to increase bioavailability by 14x
- Patented process, with micron-sized particles
- Toxicity study to confirm the safety
- NDI & GRAS in process
- Stable in pH 2.4 and 7 water solution for 28 days
- Shelf-life 24 months
Applications & Uses
- Applications
- Dosage Form
- Food & Nutrition Applications
- Use Level
- Recommended Dosage : 200 mg
- Dosage and Forms
CuminUP60® can be used in capsule, tablet, beverage, gummy form, and more.
It can be used alone, or formulated with other nutrients.
Our small one-a-day-dose at 200 mg packs a punch to bring you maximum health benefits at maximum bioavailability.
Properties
- Solubility
- Typical Properties
| Value | Units | Test Method / Conditions | |
| Potency | 60.0 | % | — |
| Total Curcumin Content | 120.0 | mg | — |
Regulatory & Compliance
- Certifications & Compliance
Technical Details & Test Data
- Human Clinical Study on Oral Bioavailability (Pharmacokinetic Study)
- The study utilizes a single equivalent dose, randomized design with crossover to compare the relative bioavailability between CuminUP60® and regular 95% curcumin.
- Twelve healthy men and women (18 to 55-year-old) were enrolled, and twenty blood samples were obtained from each subject over 72 hours.
- CuminUP60® delivered significantly higher plasma curcumin (Sum of curcuminoids and their relative glucuronide and sulfate metabolites) concentrations by 14 times, compared to the regular 95% curcumin.
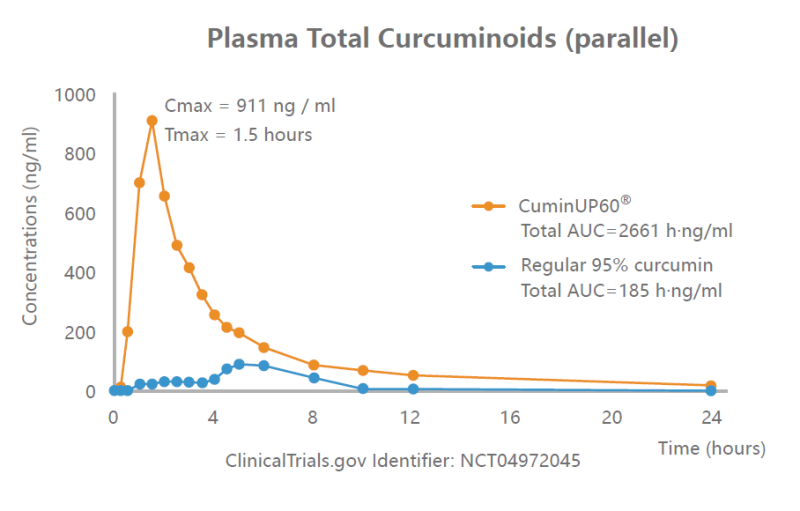
Significant increase in the concentration of total curcuminoids in serum from baseline to 1.5 hours after a single dose of CuminUP60®.
- Pre-Clinical Efficacy Studies on Efficiency of absorption
CuminUP60® has an enhanced cell intake of curcumin and increased cell
membrane fluidity in our cell fluorescence studies. Cell intake of regular 95% curcumin and CuminUP60®.
Cell intake of regular 95% curcumin and CuminUP60®.
Under the same fluorescence parameters, we observed the intensity of green fluorescence to reflect the uptake of curcumin by Caco-2 cells.- Pre-Clinical Efficacy Studies on Inhibition of breast cancer cells
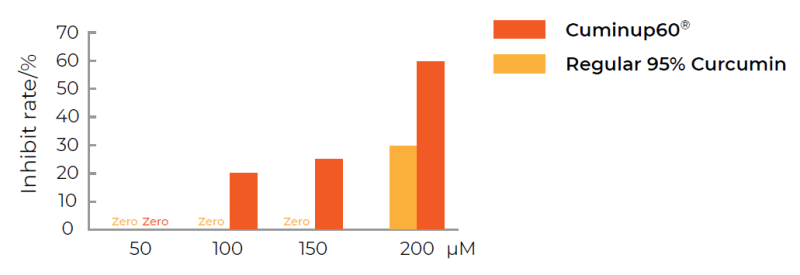
Significant increase in the proliferation inhibition rate of cancer cells with the increased concentration of CuminUP60®.
- Pre-Clinical Efficacy Studies on Inhibition of tumor growth
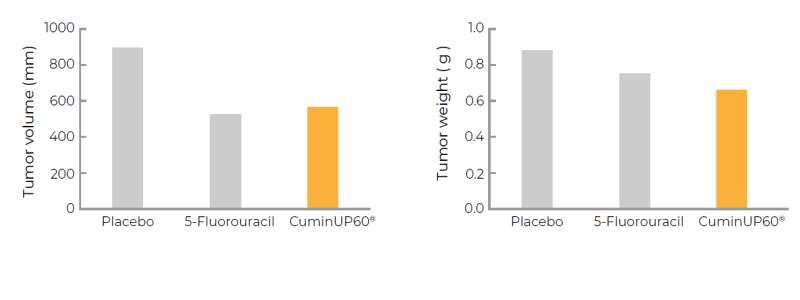
Significantly inhibit the increase in tumor volume and weight in nude mice, at a clinical dosage of 200 mg per day. 5-Fluorouracil: positive drug for tumor treatment.
- Pre-Clinical Efficacy Studies on Joint Health
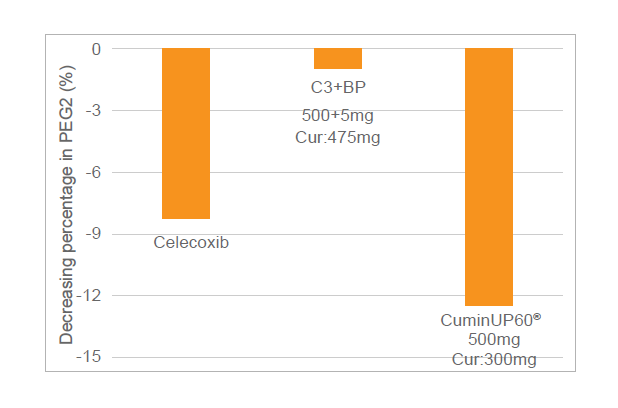
Significantly decrease the expression of inflammatory factors, such as PEG2, MMPs, COX-1, COX-2, PGE2, IL-6, superior to C3 Complex®+1% piperine.
- Pre-Clinical Efficacy Studies on Immune Health
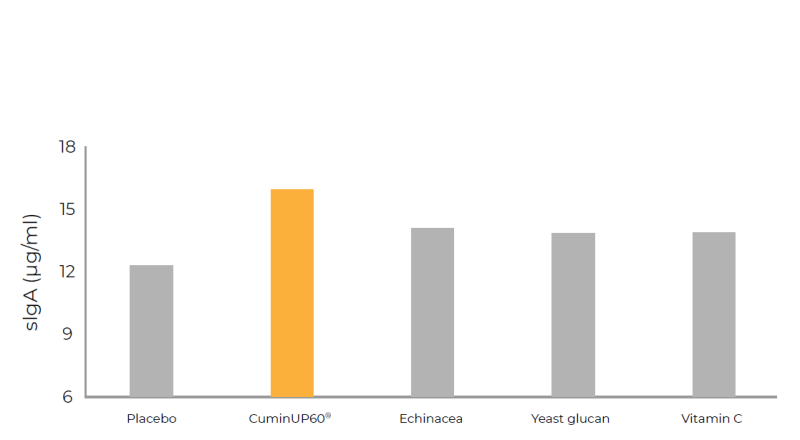
CuminUP60® increased the secretion of IgG, IgM and sIgA, superior to Echinacea extract, Yeast glucan (Wellmune®) and Vitamin C.It also inhibited the expression of proinflammatory factors, such as IL-6, IL-12, IL-1ß and IFN-γ.
- Stability Studies
- CuminUP60® can be used in capsule, tablet, beverage, gummy form, and more.
- It can be used alone, or formulated with other nutrients.
- Just 200 mg a day will give your health and wellness.
a) In water solution with various pH values
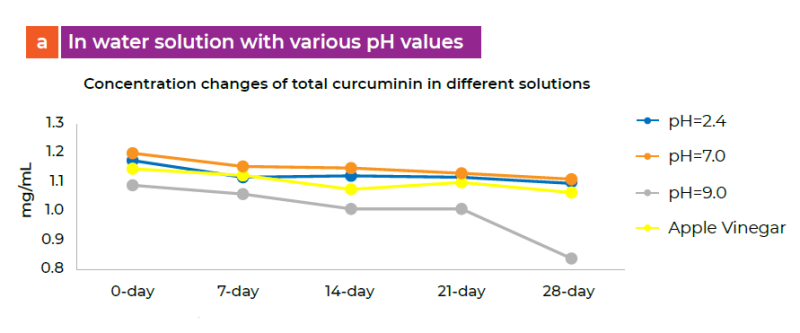
The data showed that CuminUP60® is stable at various pH levels at 2.4, 7.0 and vinegar. The concentration of total curcumin and solution color were not changed significantly during the study. However, there was a sharp decrease in the concentration of total curcumin and change in color of the solution over time at pH 9.
b) In heat sterilization of the solutions
The solutions were sterilized in 121 °C for 15 min.
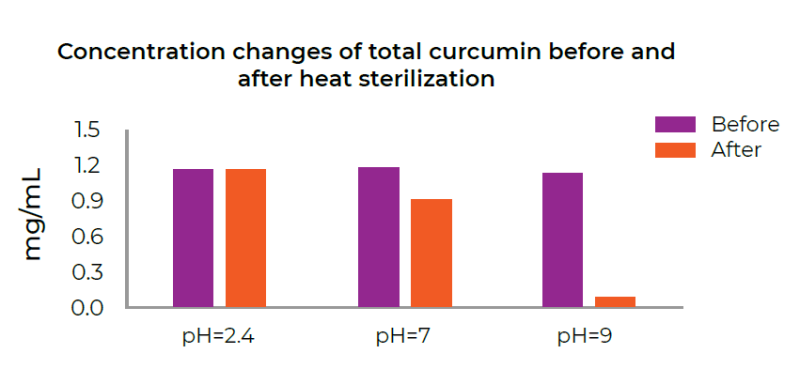
The data showed that CuminUP60® was stable in the acidic solution while it degraded by 23% and 92% at pH 7 and 9, respectively.
Storage & Handling
- Shelf Life
- 24 months

