Knowde Enhanced TDS
Identification & Functionality
- Ingredient Name
- Ingredient Origin
- Pharma & Nutraceuticals Functions
- Ingredients
- Eucommia Extract, Drynaria Extract, Chinese Dodder Extract
Features & Benefits
- Benefit Claims (Health)
- Labeling Claims
- Food Ingredients Features
- Product Background
- EuBone® is a patented formulation of standardized extracts of Eucommia ulmoides Oliv barks and leaves, Drynaria fortune (Kunze) J. Sm rhizomes, and Cuscuta chinensis Lam seeds.
- It can help manage women’s hormones and bone health, and also has been submitted to FDA for NDI Notification in USA and EFSA for the Novel Food application in Europe.
- Product Highlights
- Clinically Proven
- Regulates Calcium and Phosphorus Metabolism
- Balances Osteoblasts and Osteoclasts Formation
- Established Mechanism of Action
- 100% Herbal Formula
- Developed Based on 2,000-Year Traditional Chinese Medicine History
- Allergen-Free, Kosher, Halal
- Patents That Cover Manufacturing, Composition and Application
- Toxicity Study to Confirm the Safety
Applications & Uses
- Markets
- Dosage and Forms
Amount Per Serving % Daily Value EuBone® Blend (Eucommia ulmoides bark and leaf extract,
Drynaria rhizome extract, Chinese Dodder seed extract)500 mg Dosage and Target Population
- 1-2 serving per day.
- EuBone® is suitable for peri & post-menopausal women, people with osteoporosis and prone to bone fracture.
Application
EuBone® can be formulated in both capsule and tablet form along with minerals and vitamins to support long-term bone health and hormonal balance.
Regulatory & Compliance
- Certifications & Compliance
Technical Details & Test Data
- Human Clinical Study
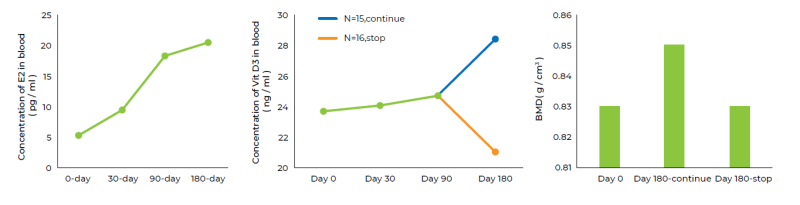
- A 180-day, single-center, open-label human clinical study was conducted to prove the efficacy and safety of EuBone®.
- The subjects aged between 50 and 80 years old with mild to moderate osteoporosis were enrolled.
- The results showed that EuBone® formulation can significantly increase the estradiol level in postmenopausal women by 2.9 times, and further promote their bone health by rising Bone Mineral Content (BMC), Bone Mineral Density (BMD) by 2.4%.
- Hematology, urine, blood pressure and electrocardiogram results showed no significant differences before and after the treatment, indicating the safety of EuBone®.