Knowde Enhanced TDS
Identification & Functionality
- Chemical Name
- Ingredient Name
- Vitamin Type
- Animal Feed & Nutrition Functions
- Food Ingredients Functions
- Pharma & Nutraceuticals Functions
- Molecular formula
- C₆H₇NaO₆
- Product Families
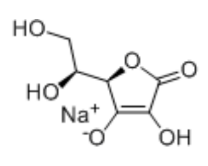
- Structural Formula
Features & Benefits
- Labeling Claims
- Food Ingredients Features
Applications & Uses
- Applications
- Dosage Form
- Food & Nutrition Applications
- Product Applications
Sodium Ascorbate is used as ascorbic acid supplementary agent to supplement the deficiency of ascorbic acid in taking, protect food and drinks from the off-color and off-taste. It can be used for pressing oral and chewing tablets directly and can be used for pressing vitamin complex and complex tablets of vitamin and mineral substance.
Properties
- Solubility
- Soluble in
- Water
- Insoluble in
- Trichloromethane, Sulphuric ether
- Slightly Soluble in
- Ethyl alcohol
- Typical Properties
- Microbiological Values
| Value | Units | Test Method / Conditions | |
| Relative Molecular Mass | 198.1 | — | — |
| Specific Optical Rotation | 103 - 108 | ° | — |
| pH Value (10% W/V) | 7.0 - 8.0 | — | — |
| Loss on Drying | max. 0.25 | % | — |
| Assay (On Dried Basis) | 99.0 - 101.0 | % | — |
| Clarity of Solution | Clear | — | — |
| Color of Solution | max. BY₆ | — | — |
| Impurity E | max. 0.3 | % | — |
| Impurity C | max. 0.15 | % | — |
| Impurity D | max. 0.15 | % | — |
| Unspecified Impurities | max. 0.10 | % | — |
| Total of Impurities Other Than C and D | max. 0.2 | % | — |
| Heavy Metal Content | max. 10 | ppm | — |
| Copper Content | max. 5 | ppm | — |
| Iron Content | max. 2 | ppm | — |
| Nickel Content | max. 1 | ppm | — |
| Cadmium Content | max. 1 | ppm | — |
| Mercury Content | max. 1 | ppm | — |
| Lead Content | max. 2 | ppm | — |
| Arsenic Content | max. 3 | ppm | — |
| Total Plate Count | max. 1000 | cfu/g | — |
| Yeast & Mold Count | max. 100 | cfu/g | — |
| Staphylococcus Aureus | Negative | — | — |
| Value | Units | Test Method / Conditions | |
| Escherichia Coli | Negative | — | — |
| Salmonella | Negative | /10g | — |
Regulatory & Compliance
- Certifications & Compliance
Packaging & Availability
- Packaging Information
Sodium Ascorbate bulk materials are packed in a two-layer bag, and the inner transparent plastic bag is thermal sealed, the outer bag is aluminum foil bag. Both are vacuum sealing.
Then pack in a carton box (or drum), sealed with adhesive tape (or lead sealing).
Storage & Handling
- Stability
Sodium Ascorbate is not stable in the air; the color will become faint when exposed to the light.
- Storage Information
- Store in sealed containers and keep in a dry place and away from light.
- At least 24 months from date of manufacturing provided the containers are unopened and stored under the above-mentioned conditions.

