Knowde Enhanced TDS
Identification & Functionality
- Active Component
- Ingredient Name
- Ingredient Origin
Features & Benefits
- Benefit Claims (Health)
- Labeling Claims
- Prostatic Ailment
Prostatic ailments are among the most common urologic problems in adult males. BPH (benign prostatic hyperplasia) is the third most frequent urologic diagnosis in men over 50.
although prostatitis can also affect young people, especially sportsmen and cyclists. Prostatic ailments share inflammation and oxidative stress as risk factors and cause of chronicty.Epilobium angustifolium L (Onagraceae) is a well-known European plant traditionally used for prostatic ailments such as prostatitis and BPH ENOTprost isa dry extract with a very rich phytocomplex containing more than 15% of Oenothein B It is a strong anti-inflarmmatory and antioxidant ingredient for innovative food supplements
Applications & Uses
- Applications
- Dosage Form
- Physiologic and Healthcare Applications
- Anti-inflammatory.
- Antioxidant.
- Anti-prostatic (also used in BPH).
Properties
- Odor
- Characteristic
- Taste
- Characteristic
- Partially Soluble in
- Water
- Typical Properties
- Microbiological Values
- Nutritional Information
- Heavy Metals
| Value | Units | Test Method / Conditions | |
| Particle Size (through 300 microns) | 90 | % | — |
| Oenothein B | min. 15 | % | — |
| Bulk Density | 300 - 600 | g/L | — |
| Loss on Drying | max. 7.0 | %w/w | — |
| pH | 4 - 6 | — | — |
| Heavy Metals | max. 20 | ppm | (method C Ph. Eur. current edition) |
| Aflatoxin B1 | max. 2 | ppb | — |
| Aflatoxin B1,B2,G1,G2 | max. 4 | ppb | — |
| Benzo(a)pyrene | max. 10 | ppb | — |
| Sum of Benzo(a)pyrene, benzo(a)anthracene, benzo(b)fluoranthene and chrysene | max. 5 | ppb | — |
| Pyrrolizidine Alkaloids | max. 400 | ppb | — |
| Bile-tolerant gram-negative bacteria | max. 100 | ufc/g | — |
| Value | Units | Test Method / Conditions | |
| Bacterial Count | max. 50000 | ufc/g | (TAMC: ref. 5.1.8, cat. B oral use): |
| Yeasts and Moulds Count | max. 500 | ufc/g | (TYMC: ref. 5.1.8, cat. B oral use): |
| Salmonella | Absent in 25g | — | (ref. 5.1.8, cat. B oral use): |
| Escherichia coli | Absent in 1g | — | — |
| Value | Units | Test Method / Conditions | |
| Carbohydrates | 90 - 95 | % | — |
| Fat Content | 0 - 1 | % | — |
| Protein Content | 0 - 1 | % | — |
| Energy Value | 409 | Kcal/100 gr | — |
| Value | Units | Test Method / Conditions | |
| Minerals | 3 - 5 | % | — |
| Lead Content | max. 3 | ppm | (ref. Reg. (EC) 1881/2006) |
| Cadmium Content | max. 1 | ppm | (ref. Reg. (EC) 1881/2006) |
| Mercury Content | max. 0.1 | ppm | (ref. Reg. (EC) 1881/2006) |
Regulatory & Compliance
- Certifications & Compliance
- Certifications
GMO: Free from GMO (Reg. (EC) 1829/2003 and 1830/2003) BSE/TSE FREE - GLUTEN FREE
ALLERGENS: Free from substances or products causing allergies or intolerances (Reg. (EU) 1169/2011 Annex II)
Technical Details & Test Data
- Metabolic Profile
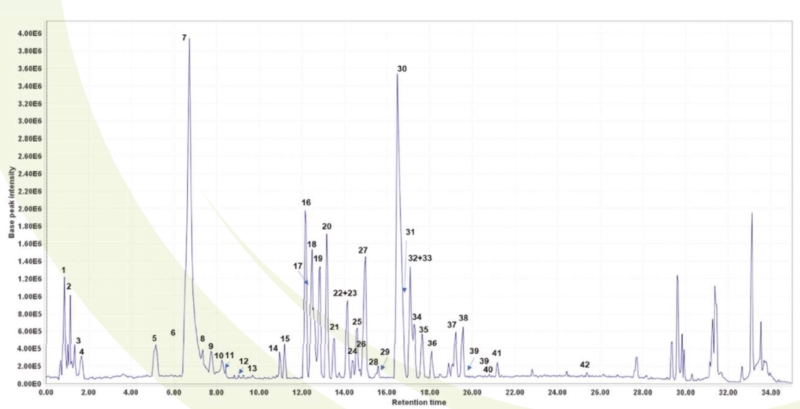
The metabolic profile of ENOTprost was analyzed by means of UHPLC- LTQ Orbitrap and 42 compounds were identified: 8 organic and phenolic acids, 1 Sugar 1 tannin. 3 ellagitannins and 29 flavonoids. Moreover. miquelianin, the major flavonoid glycoside that only characterizes the E angustifolium species, was found (Esposito et al, 2021).
The bioaccessibility and bioavailabilüity studies suggested the use of a gastro-resistant dosage form. since polyphenols suffered from dogradation after both oro-gastric and duodenal digestion processes (Dacrema et al, 2020).- Clinical Trail
A monocentric, randomized, double-blind, placebo-controlled clinical trial was conducted on 128 Italian volunteers with BPH to demonstrate the effects of ENOTprost.
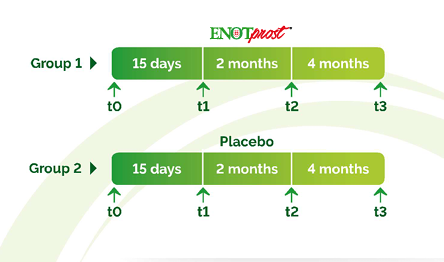
The Intermational Prostate Specific Score (PSS) is a validated questionnaire to assess
BHP symptoms in men with urinary complaints. The score can range from 0 to 35.
Indicating asymptomatic to very symptomatic subjects. The results are represented
by arrows to indicate generally the QoL (Quality of Life) of participants.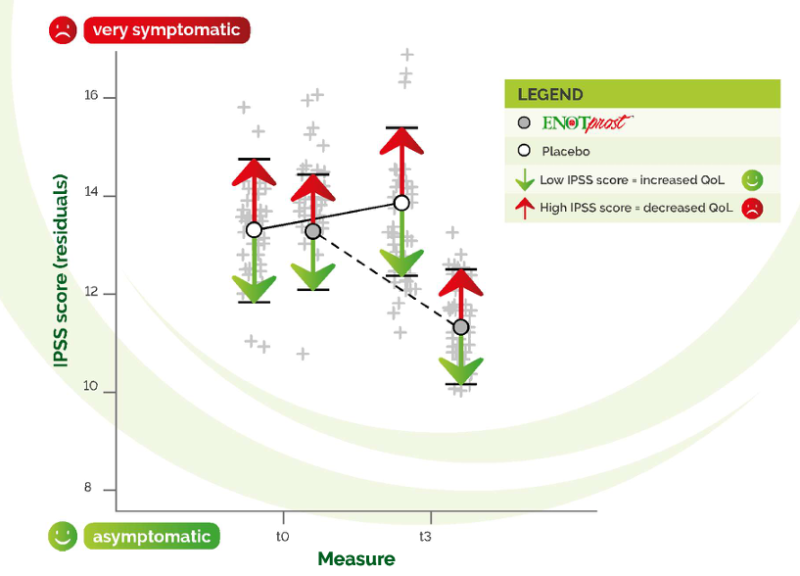
IPSS score significantly decreased by nearly 2 points between t0 and t3 in the
ENOTprost" treated group and slightly increased (06 points) in the placebo group
showing an improvement in the quality of life of the subjects treated with the
ENOTprost and highlighting the protective effect of this supplementation. Subjects with BPH may have difficulties in bladder emptying The narrowing of the urethra may cause acute/chronic urinary retention, which is the most important complication associated with BPH The bladder post-void residual volume (PVR) was monitored by prostate ultrasound to assess the efficiency of bladder emptying.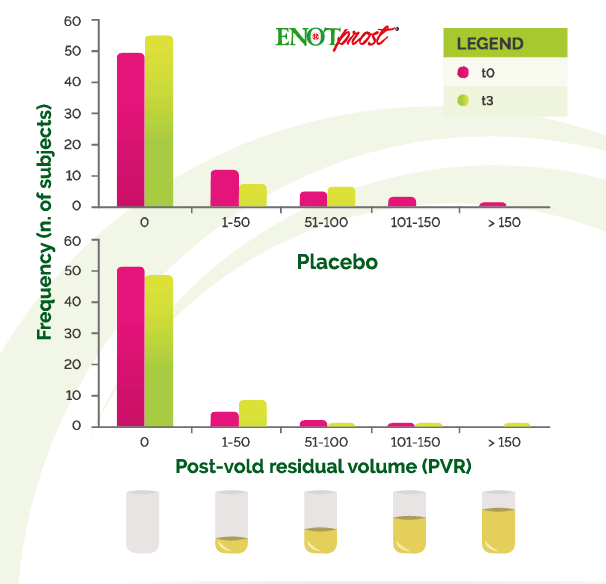
In the ENOTprost group the number of subjects with a Low residual urine volume in the bladder significantly increased. while there was a decreasing in the number of subjocts with residual urine volume higher than 100 ml.
Nocturia (the need to urinate two or more times per night) is a serious problem
With a high impact on the quality of sleep. leading to sleep disorders, decreased
quality of life and depression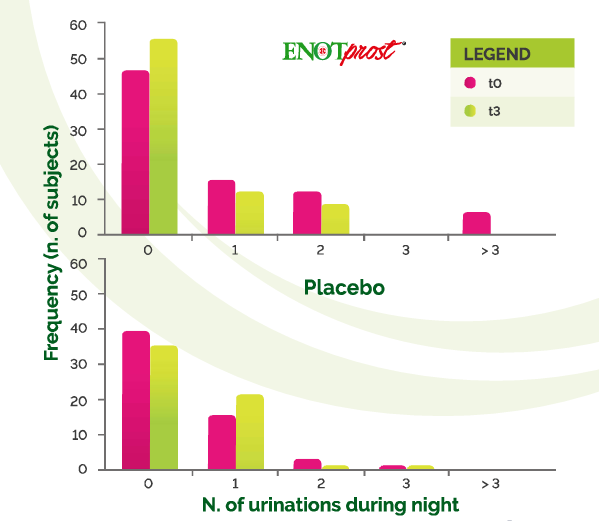
In the ENOTprost group. the frequency of subjects without urination over night increased by whereas it 217% decreased by 10.2% in the placebo group
Moreover, the number of subjects urinating three or more times per night was
Completely wiped out in the treated group but remained unchanged in the place group
Storage & Handling
- Shelf Life
- 2 Years
- Storage
Store in a well closed container away from moisture and direct sun light.

