Knowde Enhanced TDS
Identification & Functionality
- Active Component
- INCI Name
- Ingredient Origin
- Cosmetic Ingredients Functions
- CAS No.
- 90028-28-7
- EC No.
- 289-817-3
- Technologies
Features & Benefits
- Benefit Claims
- Labeling Claims
- Key Features
- Not irritating
- Excellent antioxidant (oxidant scavenging) potential
- Excellent demonstrated transdermal diffusion.
- Helps to promote collagen, elastin and AQP-3 gene expression.
- Protects skin cells against blue light
- Protects skin cells against pollution
- Product Benefits
Patented Natural Amla Berry Source
100% Vegan and known allergen & GMO-FreeSustainably Harvested
Solar powered extractors, 250K liter well rain water harvest & zero effluent dischargeSuperior Transdermal Delivery
Near 8x the penetration of standard Vitamin C
Best-in-Class Skincare Performance
Outperforms leading skincare actives & antioxidants
Transformative Technology
First ever Vitamin C+ Ellagic Acid natural polyphenol complex
Globally Compliant & Desirable INCI
Phyllanthus emlica fruit extract
Multi-benefit & Deeper Penetrating Activity
Boosts collagen & elastin expression & powerfully protects against environmental aging factors.
Applications & Uses
- Markets
- Bath & Shower Applications
- Skin Care Applications
- Use Level
- 0.2 - 0.25 (Facial cleanser & cleansing gel), 0.2 – 0.25 (Facial & scalp serum and cream), 0.2 – 0.25 (Facial mask)
- Formulation Procedures
While mixing, add C-Cos powder into final phase of water-based formulation when temperature is under 40C / 104F.
- Applications
- Facial and / or body gels.
- Facial and / or body creams.
- Facial serums
- Solutions containing surfactants.
- Facial and / or body masks
Properties
- Odor
- Characteristic
- Appearance
- Hygroscopic powder
- Soluble in
- Water, Glycerin, Propylene Glycol
- Physical Properties
- Analytical Properties
- Active Ingredient
- Microbiological Values
- Heavy Metals
| Value | Units | Test Method / Conditions | |
| Identification | Pass | - | TLC |
| Value | Units | Test Method / Conditions | |
| Carrier Used | None | - | In House Specification |
| Excipients | None | - | In House Specification |
| Extraction Solvent (Water) | 100 | % | In House Specification |
| Herb Extract Ratio | 25:1 | - | In House Specification |
| Moisture | max. 5 | % | USP 37 <921> |
| Particle Size (Through 30 Mesh) | 100 | % | USP 37 <786> |
| Pesticide Residue | Complies with USP | - | USP 37 <561> |
| pH (1% Solution) | 2.5 – 4.5 | - | In House Specification |
| Residual Solvent | None | - | USP<467> |
| Solubility (in water, 2% solution) | Soluble completely | - | IP |
| Tap Density | 0.50 - 0.80 | g/ml | USP 37 <616> |
| Value | Units | Test Method / Conditions | |
| Ellagic Acid (after Hydrolysis) | min. 10 | % | HPLC |
| Hydrolysable GalloEllagic Tannins | min. 25 | % | HPTLC |
| Total Polyphenol Content | min. 35 | % | Titration |
| Vitamin C Content | 1 - 1.5 | % | Titration method |
| Value | Units | Test Method / Conditions | |
| Coliform | Absent | per 10g | AOAC, BAM |
| E.coli | Absent | per 10g | AOAC, BAM |
| Molds and Yeast | max. 100 | cfu/g | AOAC, BAM |
| Salmonella | Absent | per 25g | AOAC, BAM |
| Total Plate Count | max. 100 | cfu/g | AOAC, BAM |
| Value | Units | Test Method / Conditions | |
| Arsenic Content | max. 1 | ppm | ICP-MS |
| Cadmium Content | max. 1 | ppm | ICP-MS |
| Lead Content | max. 3 | ppm | ICP-MS |
| Mercury Content | max. 0.1 | ppm | ICP-MS |
| Total Heavy Metals | max. 10 | ppm | ICP-MS |
Regulatory & Compliance
- Certifications & Compliance
- Chemical Inventories
- C-Cos™ Regulatory Statement
United States
Currently acceptable for sale and use in formula.
European Union
No limitations according to the EEC Cosmetic Directive 76/768/EEC; currently acceptable for cosmetic use.
Japan
Phyllanthus emblica fruit extract is not specifically regulated according to the Ministry of Health, Labor and Welfare (MHLW) Cosmetic Standards. Currently acceptable for use for general cosmetic formulation.
South Korea
Currently acceptable for use under MFDS Cosmetics Act as a general cosmetics ingredient.
United Kingdom
Currently acceptable for use in topical cosmetic formulas
Canada
Phyllanthus emblica fruit extract currently allowable for topical use under Food & Drug Act. Does not appear on Canadian banned ingredient Hot List.
Brazil
Currently acceptable for use and not included on ANVISA's restricted or banned ingredients list
China
Phyllanthus emblica fruit extract currently listed in IECIC 2021 as # 08191. Currently acceptable for use.
Australia
Phyllanthus emblica fruit extract not currently listed in the Industrial Chemicals Inventory. Meets requirements of “Low Risk” ingredient, and is acceptable for use and sale (customer should check if registration with AICIS will be required).
- Product Statement
REACH Status
Arjuna Natural confirms that C-Cos™, is a 100% natural ingredient, processed without solvents or chemical additives. It fully meets REACH Naturals definition.
Cosmetic status
Arjuna Natural confirms that C-Cos™, is suitable for cosmetic application, it is not included in the list of substances prohibited in cosmetic products. Neither does it contain parabens, restricted preservatives, colorants or UV filters (Annex II-VI of EU Regulation 1223/2009).
Kosher
Arjuna Natural confirms that C-Cos™ is not Kosher certified
Halal
Arjuna Natural confirms that C-Cos™ is NOT Halal certified, however it does not contain any animal derived product or ingredient.
Calif Proposition 65
Arjuna Natural confirms to their best of their best of their knowledge that C-Cos™, does not contain any contaminants or bi-products known to the state of California that may cause cancer or reproductive toxicity as listed under proposition 65 State Drinking Water & Toxic Enforcement Act.
Animal testing
Arjuna Natural confirms that C-Cos™ has not been Animal Tested for cosmetic purposes by or on behalf of the company, nor has any of its component parts named in the international cosmetic ingredient Dictionary & Handbook (11th Edition), 31st December 1990.
Leaping Bunny
Arjuna Natural confirms that C-Cos™ complies with the criteria of the humane cosmetics Standard & has not been tested or re-tested on animals for cosmetic purposes by or on behalf of Arjuna Natural
Vegan
Arjuna Natural confirms that C-Cos™ is suitable for Vegans.
BSE/TSE Status
Arjuna Natural confirms that C-Cos™ is not derived from Animal Origin, therefore a BSE/TSE statement is not applicable.
GMO (IP) Status
Arjuna Natural confirms that C-Cos™ is not derived/ produced from a raw material that has been genetically modified.
Gluten Free
Arjuna Natural confirms that C-Cos™ is not derived from, neither does it contain any gluten ingredients
Nanomaterials status
Arjuna Natural confirms with reference to C-Cos™, that NO nanomaterials were added at any stage of the manufacturing/production process in accordance with EU Cosmetics Regulation.
Irradiation status
Arjuna Natural confirms that C-Cos™ has NOT been irradiated at any stage of production.
Residual Solvents
Arjuna Natural confirms that NO solvents or preservatives were used in the production of C-Cos™.
PAH Status & Heavy Metals & Pesticides
Arjuna Natural confirms that there is no detectable amounts of pesticides. It is produced from natural raw material and complies within the limits of the NAOX requirements or European Regulation 1881/2006 regarding heavy metals, dioxins, dioxin-like PCBs, benzo-a-pyrene & PAH.
CMR Status
Arjuna Natural confirms that C-Cos™ is NOT classified as carcinogenic, mutagenic or toxic according to regulation 1272/2008.
CITES Status
Arjuna Natural confirms that C-Cos™ is NOT endangered & therefore not applicable for CITES certification.
Animal Testing
Arjuna Natural confirms that C-Cos™ has not harmed or inflicted any cruelty on animals or people in its production process.
HACCP & GMP Statement
Arjuna Natural confirms that C-Cos™ has been produced under Good Manufacturing Practices (GMP) and a HACCP System.
Organic
Not Applicable
Classification, labeling & Packaging (CLP)
Arjuna Natural confirms that C-Cos™ complies with Regulation (EC) N° 1272/2008; classification, labeling & packaging of substances and mixtures (CLP).
Chloramphenicol Residue
Arjuna Natural confirms that C-Cos™ is free from Chloramphenicol residues.
Palm Status
Arjuna Natural.S confirms that palm oil has not been used in the process of manufacturing C-Cos™
Parabens Status
Arjuna Natural confirms that C-Cos™ is free from parabens.
Alcohol Status
Arjuna Natural confirms that C-Cos™ is free of Alcohol.
Pyrrolizidine Alkaloids Status
Arjuna Natural confirms that C-Cos™ is free from any Pyrrolizidine Alkaloids.
SVHC (substances of Very High Concern) & Impurities Status
Arjuna Natural confirms that C-Cos™ is free from of SVHC and any impurities.
- NON-GMO STATEMENT
This is to certify that one of our products C-COSTM, under product code ACOS-35, is Non-GMO.
Technical Details & Test Data
- Third Party Testing Results
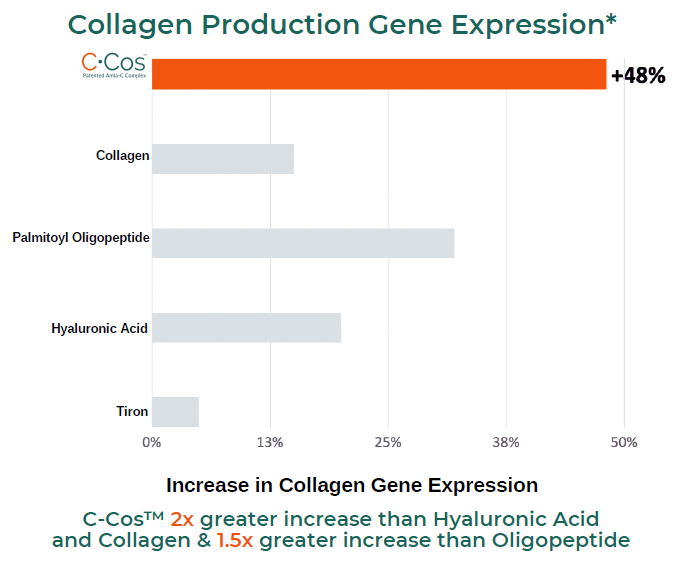
Collagen Production Gene Expression*
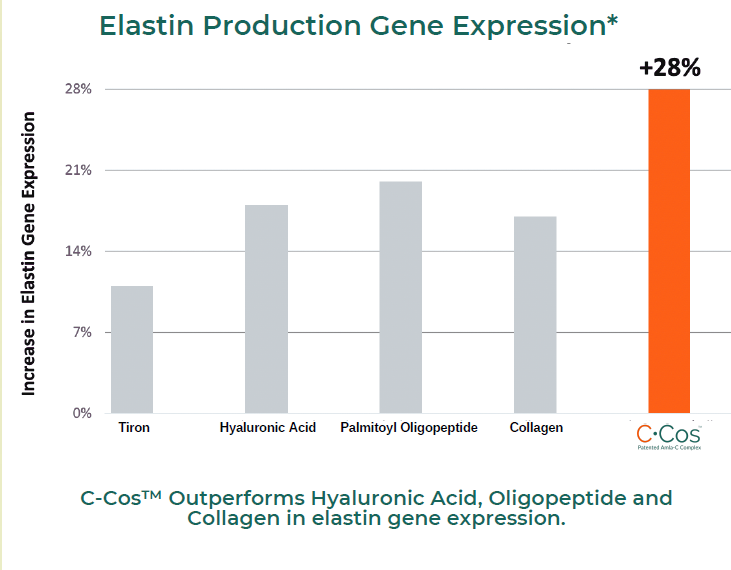
Elastin Production Gene Expression*
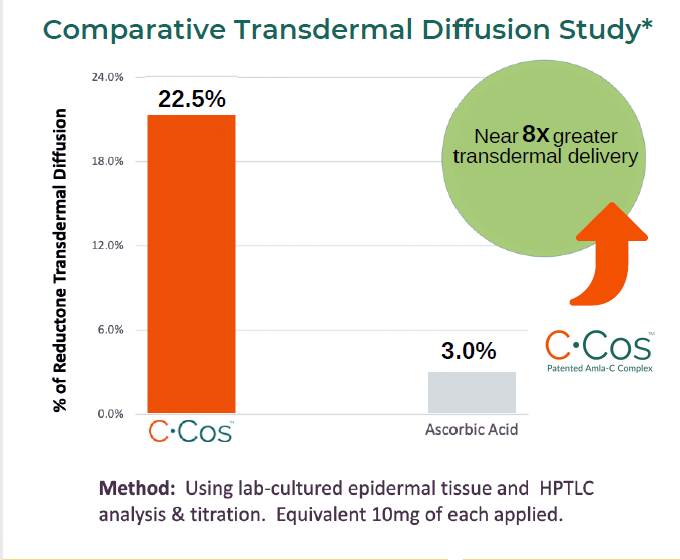
Comparative Transdermal Diffusion Study*
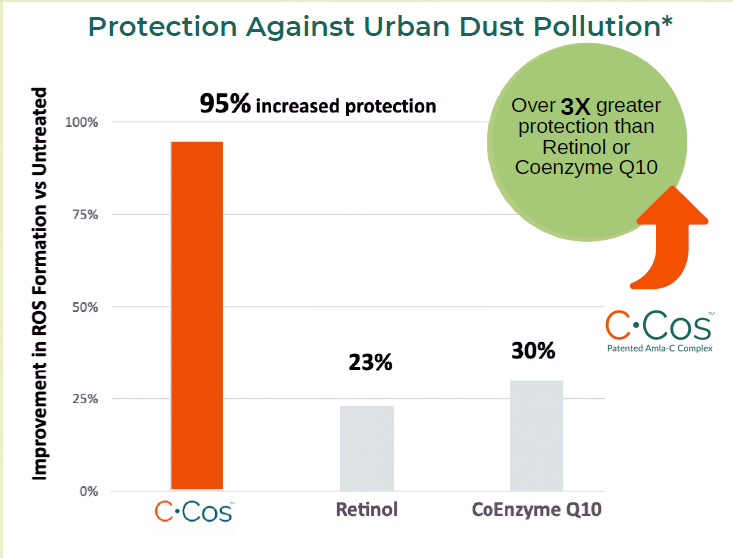
Protection Against Urban Dust Pollution*
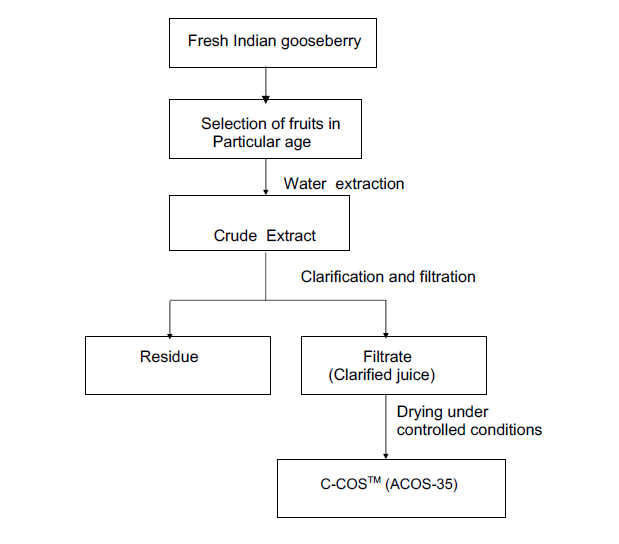
- Processing Flowchart of C-COSTM (ACOS-35)
Safety & Health
- In Vitro and Clinical Actions
PROTECTION AGAINST: Urban Dust Pollution and Blue Light
- In a 3rd party study conducted on C-Cos™, it was observed that when human keratinocytes were treated with the ingredient, ROS (reactive oxygen species) formation in response to blue light or exposure to urban dust was significantly reduced compared to untreated cells.
- For both blue light and urban dust (pollution), the reduction in ROS was found to be statistically significant.
- These results suggested that C-Cos™ is effective both in reducing ROS (reactive oxygen species) formation and in protecting against environmental aging factors. See product brochure for detailed 3rd party testing results.
GENE EXPRESSION: Collagen, Elastin and AQP-3
In a 3rd party study conducted on C-Cos™, it was observed that the ingredient significantly increased gene expression levels of collagen, elastin and AQP-3 genes in HaCaT cell lines.
TRANSDERMAL DIFFUSION
In a 3rd party university study conducted on C-Cos™ and using lab-cultured epidermal tissue, it was oserved that the ingredient significantly increased the transdermal diffusion of the Vitamin C present in the composition and transport of H+ ions.
NOT IRRITATING
C-Cos™ was 3rd party tested under occlusive dressings in 52 healthy volunteers (HRIPT). The skin reactions were evaluated by a certified dermatologist 48 and 72 hours after the occlusive application. C-Cos™ did not induce a skin primary and cumulative irritation process and did not induce a skin sensitization process in the study group, and was determined to be “Non-irritating”.
Packaging & Availability
- Packaging
1kg or 5kg HM-HDPE container with polythene inner lining
Storage & Handling
- Shelf Life
- 24 Months
- Storage Conditions
Keep in air tight containers and in inert atmosphere at ambient temperature. Always keep the original identification tag.
- Expiry Date (Expiry)
Stored in the required conditions, the shelf life is 24 months.

