Knowde Enhanced TDS
Identification & Functionality
- Ingredient Origin
- Cosmetic Ingredients Functions
- CAS No.
- 84650-00-0
- Technologies
- Product Families
Features & Benefits
- Benefit Claims
- Labeling Claims
- Key Features
- Not irritating
- Excellent antioxidant (oxidant scavenging) potential
- Protects skin cells against UVB rays
- Protects skin cells against blue light
- Protects skin cells against pollution
Applications & Uses
- Markets
- Bath & Shower Applications
- Skin Care Applications
- Use Level
- Facial cleansing gel: 10 – 15%, 10 – 15 (Facial cleansing gel), 6 – 8 (Facial serum and cream), min. 20 (Facial mask)
- Formulation Procedures
Incorporate in a formulation at 40°C max, during cooling phase or at the end of the preparation.
- Application
- Facial and / or body gels.
- Facial and / or body creams.
- Facial serums
- Solutions containing surfactants.
- Facial and / or body masks
Properties
- Physical Form
- Appearance
- Homogeneous and slightly viscous concentrate liquid
- Odor
- With fruity notes, no strange smells
- Soluble in
- Water, Glycerin, Propylene Glycol
- Physical Properties
- Microbiological Values
| Value | Units | Test Method / Conditions | |
| Foreign Material | None | — | Paired Comparison Test |
| pH | 3.8 – 4.2 | — | Digital Potentiometer (Peachmeter) |
| Total Polyphenols | 1600 (± 150) | mg eq. GAE / 100g | AOAC 2017.13 |
| Value | Units | Test Method / Conditions | |
| Aerobic Microorganisms Mesophiles | max. 100 | cfu/g | AOAC 990.12 |
| E. Coli | max. 10 | cfu/g | AOAC 991.14 |
| Molds and Yeast | max. 50 | cfu/g | AOAC 2014.05 |
| Staphylococcus aureus | Negative | per 10g | AOAC 2003.07 |
Regulatory & Compliance
- Certifications & Compliance
- Chemical Inventories
- Product Statement
REACH Status
Sanam Company S.A.S confirms that Naox® Derma, is a 100% natural ingredient, processed without solvents or chemical additives. It fully meets REACH Naturals definition.
Cosmetic status
Sanam Company S.A.S confirms that NAOX® Derma, is suitable for cosmetic application, it is not included in the list of substances prohibited in cosmetic products. Neither does it contain parabens, restricted preservatives, colorants or UV filters (Annex II-VI of EU Regulation 1223/2009).
Kosher
Sanam Company S.A.S confirms that NAOX® Derma is Kosher certified
Halal
Sanam Company S.A.S confirms that NAOX® Derma is NOT Halal certified, however it does not contain any animal derived product or ingredient.
Calif Proposition 65
Sanam Company S.A.S confirms to their best of their best of their knowledge that NAOX® Derma, does not contain any contaminants or bi-products known to the state of California that may cause cancer or reproductive toxicity as listed under proposition 65 State Drinking Water & Toxic Enforcement Act.
Animal testing
Sanam Company S.A.S confirms that NAOX® Derma has not been Animal Tested for cosmetic purposes by or on behalf of the company, nor has any of its component parts named in the international cosmetic ingredient Dictionary & Handbook (11th Edition), 31st December 1990.
Leaping Bunny
Sanam Company S.A.S confirms that NAOX® Derma complies with the criteria of the humane cosmetics Standard & has not been tested or re-tested on animals for cosmetic purposes by or on behalf of Sanam Company S.A.S
Vegan
Sanam Company S.A.S confirms that NAOX® Derma is suitable for Vegans.
BSE/TSE Status
Sanam Company S.A.S confirms that NAOX® Derma is not derived from Animal Origin, therefore a BSE/TSE statement is not applicable.
GMO (IP) Status
Sanam Company S.A.S confirms that NAOX® Derma is not derived/ produced from a raw material that has been genetically modified.
Gluten Free
Sanadores Ambientales S.A.S confirms that NAOX® Derma is not derived from, neither does it contain any gluten ingredients
Nanomaterials status
Sanam Company S.A.S confirms with reference to NAOX® Derma, that NO nanomaterials were added at any stage of the manufacturing/production process in accordance with EU Cosmetics Regulation.
Irradiation status
Sanam Company S.A.S confirms that NAOX® Derma has NOT been irradiated at any stage of production.
Residual Solvents
Sanam Company S.A.S confirms that NO solvents or preservatives were used in the production of NAOX® Derma.
PAH Status & Heavy Metals & Pesticides
Sanam Company S.A.S confirms that there is no detectable amounts of pesticides. It is produced from natural raw material and complies within the limits of the NAOX requirements or European Regulation 1881/2006 regarding heavy metals, dioxins, dioxin-like PCBs, benzo-a-pyrene & PAH.
CMR Status
Sanam Company S.A.S confirms that NAOX® Derma is NOT classified as carcinogenic, mutagenic or toxic according to regulation 1272/2008.CITES Status
Sanam Company S.A.S confirms that NAOX® Derma is NOT endangered & therefore not applicable for CITES certification.
Animal Testing
Sanam Company S.A.S confirms that NAOX® Derma has not harmed or inflicted any cruelty on animals or people in its production process.
HACCP & GMP Statement
Sanam Company S.A.S confirms that NAOX® Derma has been produced under Good Manufacturing Practices (GMP) and a HACCP System.
Organic
Not Applicable
Classification, labeling & Packaging (CLP)
Sanam Company S.A.S confirms that NAOX® Derma complies with Regulation (EC) N° 1272/2008; classification, labeling & packaging of substances and mixtures (CLP).
Chloramphenicol Residue
Sanam Company S.A.S confirms that NAOX® Derma is free from Chloramphenicol residues.
Palm Status
Sanam Company S.A.S.S confirms that palm oil has not been used in the process of manufacturing NAOX® Derma
Parabens Status
Sanam Company S.A.S confirms that NAOX® Derma is free from parabens.
Alcohol Status
Sanam Company S.A.S confirms that NAOX® Derma is free of Alcohol.
Pyrrolizidine Alkaloids Status
Sanam Company S.A.S confirms that NAOX® Derma is free from any Pyrrolizidine Alkaloids.
SVHC (substances of Very High Concern) & Impurities Status
Sanam Company S.A.S confirms that NAOX® Derma is free from of SVHC and any impurities.
- Naox® Derma Regulatory Statement
Europeian Union
Currently acceptable for use and sale
United States
Currently acceptable for use and sale
China
Coffea arabica fruit extract is not listed in the IECIC 2021, though per CSAR 2021 guidelines Naox® Derma meets the requirements of “low risk” general cosmetic. It is therefore currently acceptable for use and sale.
Japan
Currently acceptable for use and sale
South KoreaCurrently acceptable for use and sale
UKCurrently acceptable for use and sale
CanadaCurrently acceptable for use and sale
BrazilCurrently acceptable for use and sale
AustraliaCurrently acceptable for use and sale
Technical Details & Test Data
- Third Party Testing Results
POST-UVB CELL VIABILITY*
Increase in post-UVB exposure cell viability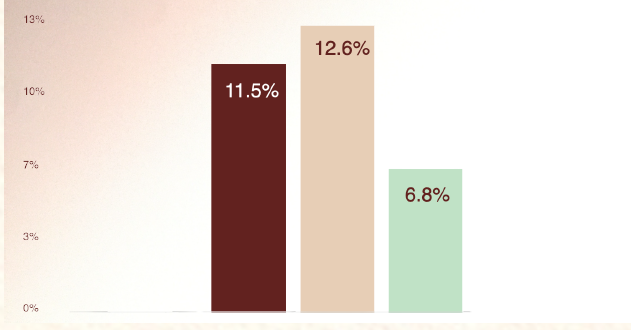

- Third Party Testing Results
BLUE LIGHT PROTECTION*
Measured reduction of ROS formation vs Untreated (RFU)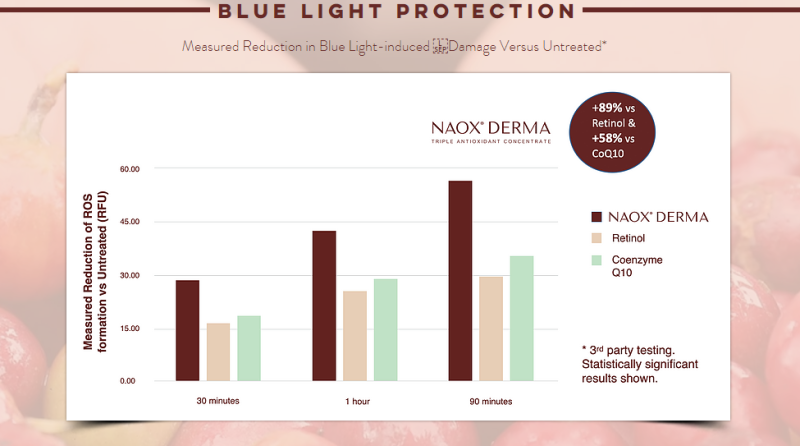
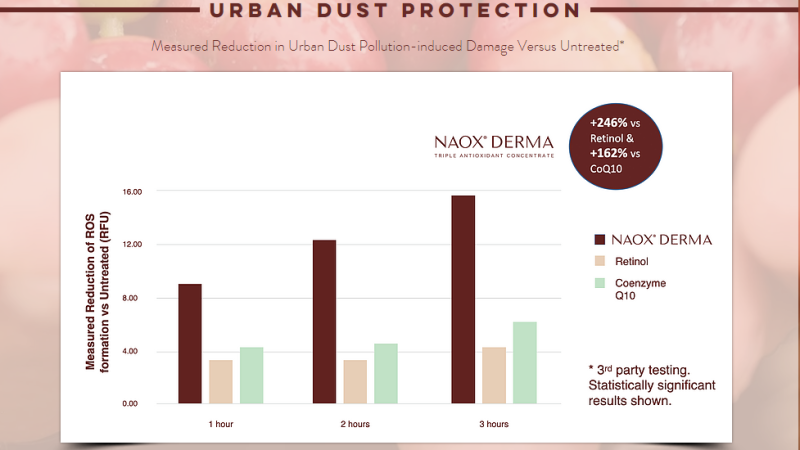
- Third Party Testing Results
URBAN DUST PROTECTION
* Measured reduction of ROS formation vs Untreated (RFU)
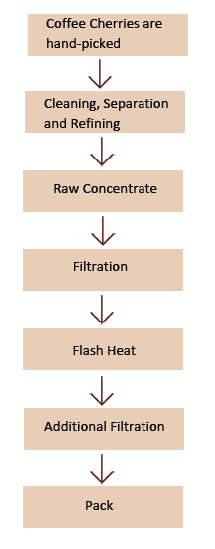
- Manufacturing Flow Chart
Safety & Health
- In Vitro and Clinical Actions
Protection against : UVB, Pollution, Blue Light
- In a study conducted on NAOX® DERMA (Coffea Arabica fruit extract) by BIOINNOVATION LABORATORIES INC, it was observed that when human keratinocytes were treated with the ingredient, ROS (reactive oxygen species) formation in response to blue light or exposure to urban dust was significantly reduced compared to untreated cells.
- For both blue light and urban dust (pollution), the reduction in ROS was found to be statistically significant.
- Regarding UVB protection, NAOX® DERMA significantly improved cell viability after UVB exposure.
- These results suggested that NAOX® DERMA (Coffea Arabica Fruit Extract) is effective both in reducing ROS (reactive oxygen species) formation and in providing UVB protection.
- See product brochure for detailed 3rd party testing results.
Not Irritating
NAOX® DERMA (Coffea Arabica Fruit Extract), was tested under occlusive dressings in 32 healthy volunteers (HRIPT). The skin reactions were evaluated by a certified dermatologist 48 and 72 hours after the occlusive application, obtaining a primary irritation index equal to 0.08, that is, "Non-Irritant".
Packaging & Availability
- Packaging Style
Commericial Offer Packing Material Exterior Packaging 1 kg flexible bag, laminated PET
sheet, coated /FLEZ 137 μ.corrugated carton box 5 kg Laminated multilayer film Flexible // coextrusion HBA (EVO-PE) 190 μ. corrugated carton box 20 kg BLUE PEBD BAG Lid Material: High Density
PolyethyleneMetal drum
Storage & Handling
- Shelf Life
- 18 Months
- Storage Conditions
Store in its original packaging at a temperature below 30⁰C, relative humidity below 70% and do not expose to direct sunlight. While not in use it must remain hermetically closed. Always keep the original identification tag.
- Expiry Date (Expiry)
- Stored in the required conditions and without uncovering, the shelf life is 18 months.
- Retest Date: 18 months

