Knowde Enhanced TDS
Identification & Functionality
- Ingredient Name
- Pharma & Nutraceuticals Functions
- Ingredients
- Turmeric Rhizome Extract
Features & Benefits
Applications & Uses
- Applications
- Food & Nutrition Applications
Technical Details & Test Data
- The LipiSperse® Advantage
Cold Water Dispersible (CWD) Powders using LipiSperse®
LipiSperse® is a dispersion technology for lipophilic (hydrophobic or water hating) powders.
It is a patented delivery system improving the bioavailability of lipophilic substances. This is due to its capacity to facilitate gastrointestinal absorption and reduce or eliminate the effect of food on the absorption of poorly water- soluble bioactive compounds. It also increases the functionality.
CWD powders have an equilibrium established between the LipiSperse® on the powder surface and the LipiSperse® in the solution.
Cold water dispersible HydroCurc® =
• Improved efficacy
• Faster onset of action
• Lower doses
• Improved user compliance.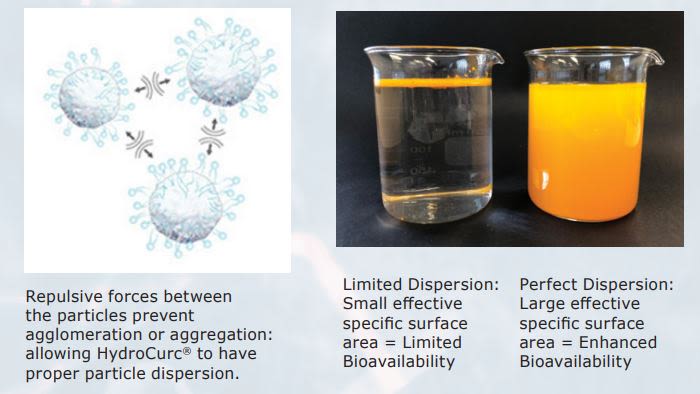
- Pharmacokinetic Study
LipiSperse® is a novel system tailored to increase the dispersion of crystalline lipophilic agents in aqueous environments. The present study aimed to compare the pharmacokinetics of a single dose of a commercially available curcumin product with a curcumin-LipiSperse® delivery system.
A single dose, randomized, double-blinded, crossed over, absorption study of curcuminoids over a 24-hour period at University of Queensland. N= 18 male & females (18- 30 years) 7 crossed over.
Single dose (750mg)
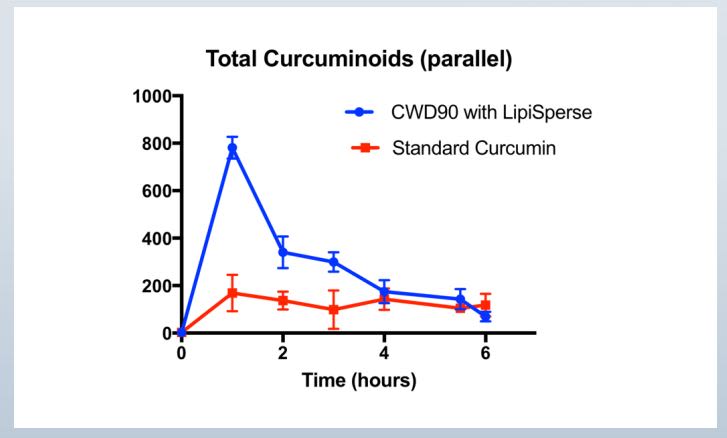
Cmax = 807 ng/ml Tmax = 1 hr Total AUC = 2,492 ng/ml P<0.05
Results :HydroCurc® delivered significantly higher plasma curcuminoid concentrations compared to the raw curcumin product.
- Exercise Recovery Study
A double-blind, randomized, placebo-controlled study was conducted to evaluate the effect of orally dosed HydroCurc® on exercise recovery in healthy males. The study was conducted with 28 recreationally trained, healthy males aged between 18 and 35 years old. Muscle fatigue was induced using leg press and completion of 4 sets for as many repetitions as possible, with 1-minute rest between sets. After muscle fatigue was induced; blood parameters, VAS pain score, thigh circumference and questionnaires were completed.
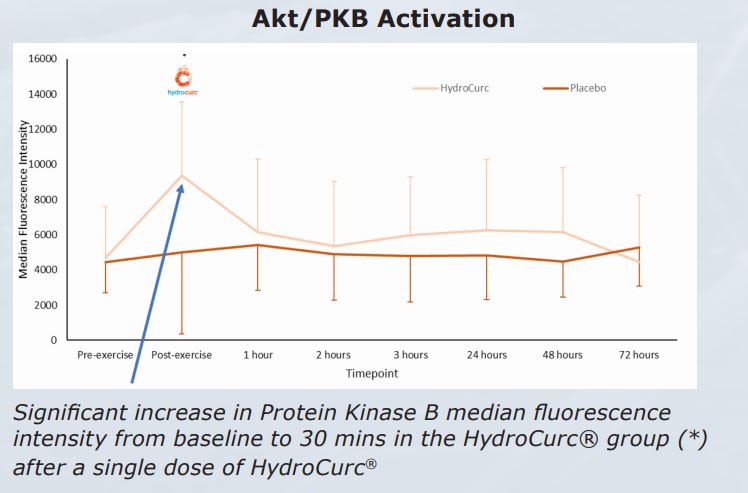 Significant increase in Protein Kinase B median fluorescence intensity from baseline to 30 mins in the HydroCurc® group (*) after a single dose of HydroCurc® Results HydroCurc® was shown to significantly:
Significant increase in Protein Kinase B median fluorescence intensity from baseline to 30 mins in the HydroCurc® group (*) after a single dose of HydroCurc® Results HydroCurc® was shown to significantly:• Lower pain score post-exercise (DOMS)
• Reduce lactate levels post-exercise
• Lower inflammatory markers (IL-6)
• Reduce thigh circumference (back to baseline)
- Cognitive Study
Brain-Derived Neurotrophic Factor (BDNF) is a critical neuroprotein that plays a structural role in the Brain and nervous system and helps support normal Brain function. BDNF supports healthy nerve cells and plays a role in growth and maintenance of neuronal cells. In the brain, BDNF is present at the connection between nerve cells and may support healthy cell to cell communications take place.
Recent research also shows that BDNF is found in regions of the brain associated with control of eating, drinking, and body weight. A 6-week double-blind, placebo-controlled, randomized study. Effects of co-administering oral iron supplementation with HydroCurc® on serum BDNF levels in healthy adults. 155 healthy adults (18-40 yrs). Outcomes measured: Change in serum BDNF levels and Change in Ferritin levels.
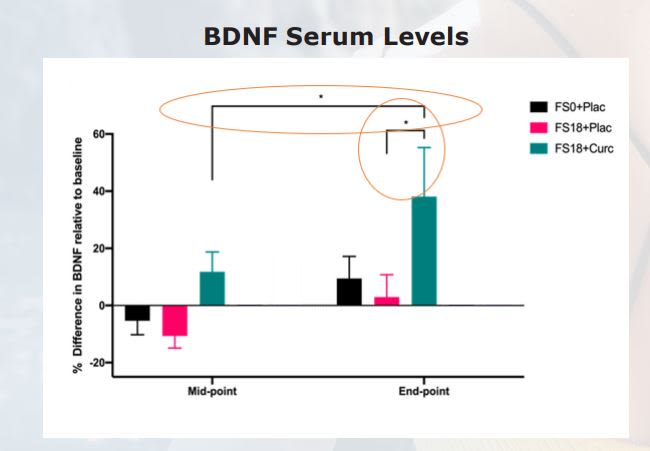
BDNF results were statistically significant between HYDROCURC + FS 18 group vs Pacebo + FS 18 group. There was also statistical significance between HYDROCURC + FS 18 group vs FS0 + Placebo group.
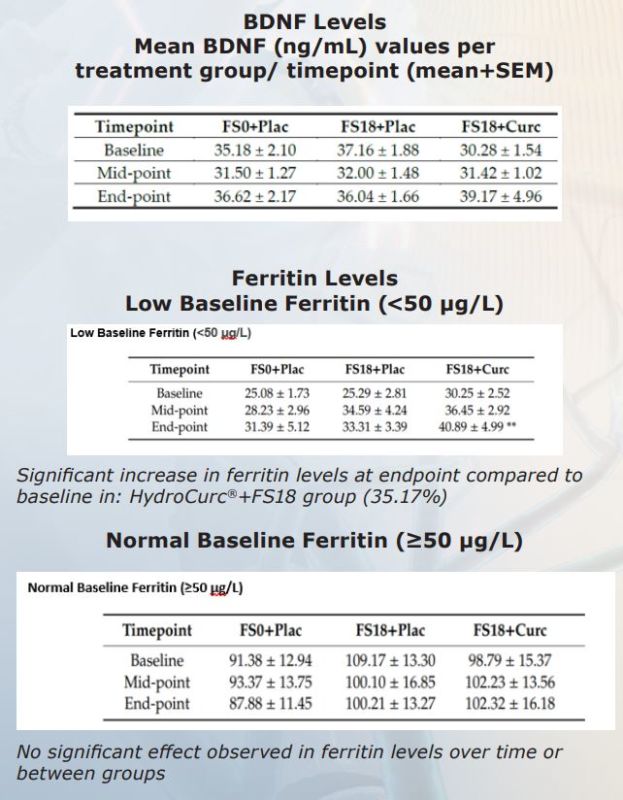
Results Co-administration of a HydroCurc® with 18 mg elemental Iron for 42 days can increase serum BDNF levels. Those with low ferritin levels may benefit from taking 500mg Hydrocurc® with 18mg Iron by: • Increasing serum BDNF levels in individuals • Enhancing ferritin formation • Correcting iron deficiency Iron deficiency can lead to impaired cognition, among other issues. Previous studies have shown that independently, curcumin and iron are associated with improved BDNF levels. In present study, co-administration shown to result in increased serum BDNF levels
HydroCurc® when combined with Iron increases the levels of BDNF
Think of brain-derived neurotrophic factor as fertilizer for your brain. You have billions of neurons (aka brain cells), and BDNF keeps them flourishing and strong. When you release BDNF, it flips the switch on a series of genes that grow brand-new brain cells and pathways. BDNF also strengthens the neurons you already have. Along with keeping you mentally alert and improving memory. As you get older, your levels of brain-derived neurotrophic factor naturally start to fall, so BDNF levels decrease with age.7
- Joint Health Study
Joint Health in an Adult Population – HydroCurc Compared to a Placebo in a randomized, double-blind study. A randomized, double-blind placebo-controlled study investigated the efficacy of HydroCurc on joint pain over a 2-week period. Group 1 – HydroCurc (40). Each participant randomized to this arm was assigned the investigational product and instructed to take 500mg once daily with water. Group 2 –Placebo (40). Each participant randomized to this arm was assigned placebo and instructed to take the product per the same instructions as the active arms.8 Change in VAS score morning and evening over 2 weeks. * significant difference between groups p<0.05
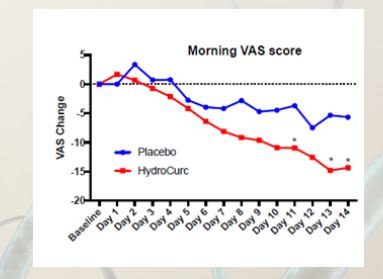
Results HydroCurc® showed relief of joint pain with a statistically significant difference compared to placebo in two weeks for morning joint pain. Fast Acting:
• Significant versus baseline in 5 days
• Significant versus Placebo in 11 days

