Knowde Enhanced TDS
Identification & Functionality
- Ingredient Name
- Pharma & Nutraceuticals Functions
- CAS No.
- 544-31-0
- Ingredients
- Palmitoylethanolamide
- EC No.
- 208-867-9
Features & Benefits
- Benefit Claims (Health)
Applications & Uses
- Applications
- Food & Nutrition Applications
Regulatory & Compliance
- Certifications & Compliance
Technical Details & Test Data
- Science Inside
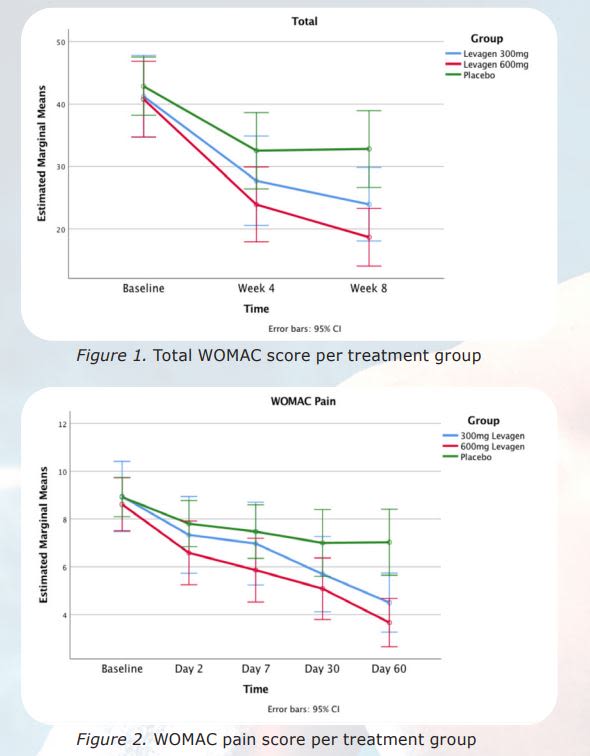
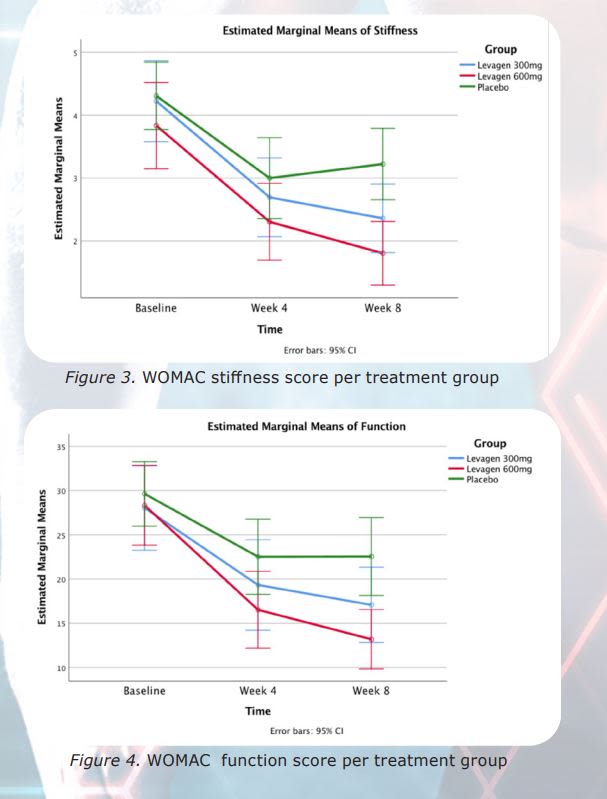
A double-blind, randomized, placebo-controlled study was conducted to demonstrate the safety and efficacy of Palmitoylethanolamide (PEA) (Levagen®) for the management of mild to moderate osteoarthritis symptoms. The study was a 120 patient study with 40 patients on placebo, 40 patients on Levagen® 300 mg a day and 40 patients on Levagen 600 mg a day. 111 patients completed the study: 35 on Levagen 600 mg, 36 on Levagen® 300 mg and 40 on placebo. The 300 mg dose was dosed 150 mg in the morning and 150 mg in the evening. The 600 mg was dosed 300 mg in the morning and 300 mg in the evening. The placebo was dosed once in the morning and once in the evening. Both the 600 mg and the 300 mg doses were found to be statistically significant compared to the placebo on the:
- total WOMAC score
- pain subdomain
- stiffness subdomain
- function subdomain
High Quality Palmitoylethanolamide (PEA):