Knowde Enhanced TDS
Identification & Functionality
- Chemical Name
- Pharma & Nutraceuticals Functions
- Molecular formula
- (C₆H₁₁NO₄)ₙ
- CAS No.
- 9012-76-4
- EC No.
- 618-480-0
- Technologies
- Product Families
- Chitosan Oligomer
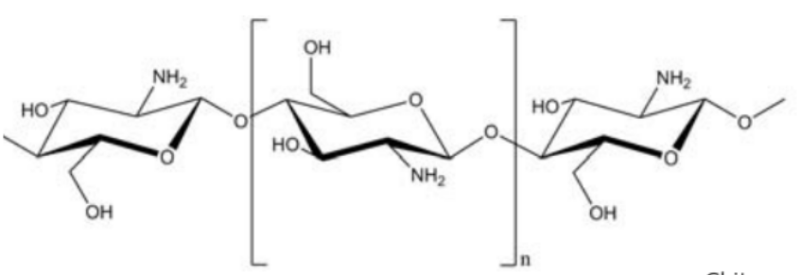
Chitosan oligosaccharide is an oligomer of β-(1 ➔ 4)-linked d-glucosamine. Chitosan oligomer can be prepared from the deacetylation and hydrolysis of chitin.
- Chemical Structure
Features & Benefits
- Benefit Claims
- Labeling Claims
- Properties
- Biodegradable by enzymes, non-toxic
- Detected in the human body as an endogenous substance
- Inhibiting the growth of bacteria and fungi
- Film- and fiber-forming, cross-linking
- Anti-inflammatory
- Haemostatic, wound-healing
- Odor-absorbing, capable of bonding to proteins, heavy metals, suspended solids
- Having a stimulating effect on the immune system and metabolism
- Properties
- Not thermo-elastic, decomposes at 280° C
- pKa 6.3
- High charge carrier density
- Biodegradable
- Non-toxic
- Bacteriostatic
- Fungistatic
- Film and fiber forming, cross-linking
- Immobilization of living organisms
- Anti-inflammatory, improves wound healing
- Stimulation of immune system and metabolism
- Deodorizing
- Bonding ability to proteins, heavy metals and aerosols
Applications & Uses
- Markets
- Applications
- Manufacturing Process
In the deacetylation process, chitin is subjected to treatment with concentrated lye to dissociate acetyl groups from the polymer. The result is chitosan that, depending on the degrees of deacetylation and polymerization (chain length), has more or less pronounced properties.
- Applications
Cosmetics industry
- Skin creams - Enhancement of water bonding capacity; bonding of perfume oils; nurturing properties (e.g. neurodermatitis)
- Hair care - Film formation on hair (protection), thus improving hair elasticity and avoidance of split ends; reduction of electrostatic charging
- Oral hygiene - Bacteriostatic effect; gel basis for toothpastes; N-carboxy methyl chitosan against periodontitis
Medical Devices
- Wound and burn treatment, inter alia also with decubitus sores; artificial skin; surgical suture material; haemostatic agent
- Contact lenses
- Antibacterial and antifungal agents in formulations
- Carrier material in tissue engineering
- Implants
- Wound care - Accelerated wound healing process, combination of chitosan and chitosan oligomers for wound dressings
- Drug delivery - Vectors for gene delivery, chitosan oligomer forms complexes with plasmid DNA – drug delivery e.g. to lungs and intestinal epithelium
- Tissue engineering - Chitosan oligomer promotes differentiation of mesenchymal stem cells to osteoblasts – formation of bone tissue
- Gene delivery
- Adjuvant in vaccines
- Hydrogels
- Antibacterial effects
Pharmaceutics
- Excipient:
- Improvement of releasing properties of active substances (controlled release and drug delivery systems for the following intake routes: oral, pulmonary, parenteral, nasal, ocular, transdermal),
- As a filler in tablets,
- As a hydrogel to increase viscosity of solutions.
- Active substance: in gene and tumor therapies in the form of chitosan derivatives.
- Derivatives
Chitin and chitosan derivatives are extremely versatile. Most of them are directly soluble in water and, depending on their derivatisation, can be applied in neutral and basic pH ranges. Thus, the derivatives have very comprehensive and customized potential applications. Our standard derivatives include:
- Chitosan HCl
- N, O-Carboxymethyl-Chitosan (CMC)
- Chitosan Lactate
- Chitosan Acetate
- Chitosan Glutamate
- Production Process
Chitosan differs from chitin in that it has free amino groups (figure 1) and is obtained by deacetylizing chitin. At a minimum deacetylization level of 70% (=amount of free amino groups in the polymer) it is considered to be chitosan. The monomer of chitosan is D-amino glucose.
Properties
- Appearance
- White to Light Yellow (Solid), Clear, Colorless to Slightly Yellowish (Solution)
- Insoluble in
- Water, Sulphuric Acid
- Soluble in
- Diluted Acids (pH - max. 6)
- Typical Properties
- Microbiological Values
- Heavy Metals
| Value | Units | Test Method / Conditions | |
| Dry matter content | min. 85 | % | HMC QK-PA-0006 |
| Degree of Deacetylation | 80 - 95 | % | HMC QK-PA-0014 |
| Viscosity (1% in Acetic Acid, 20°C) | min. 5 | mPas | HMC QK-PA-0004 |
| pH (1% in Water, 20°C) | 3 - 7 | — | HMC QK-PA-0013 |
| Ash Content (Sulphated) | max. 1 | % | HMC QK-PA-0005 |
| Insolubles | max. 2 | % | HMC QK-PA-0010 |
| Molecular Weight | approx. 30 - 200 | kDa | — |
| Protein content | max. 0.5 | % | — |
| Value | Units | Test Method / Conditions | |
| Total Bacterial Count | max. 1000 | cfu/g | HMC QK-PA-0022 |
| Value | Units | Test Method / Conditions | |
| Mercury Content | max. 0.2 | ppm | HMC QK-PA-0020 |
| Cadmium Content | max. 0.5 | ppm | HMC QK-PA-0020 |
| Lead Content | max. 40 | ppm | HMC QK-PA-0019 |
| Arsenic Content | max. 0.5 | ppm | HMC QK-PA-0020 |
Storage & Handling
- Shelf Life
- 36 Months

