Knowde Enhanced TDS
Identification & Functionality
- INCI Name
- Ingredient Origin
- Cosmetic Ingredients Functions
- Technologies
- Product Families
- Manufacturing Process
Biobased epichlorohydrin is reacted with 1-methylheptanol using SnCl4 as a catalyst to yield methylheptyl glycidyl ether as an intermediate, which is subsequently hydrolyzed using aqueous NaOH to yield methylheptylglycerin. The crude product is isolated, purified via distillation, and filtered to yield 99+% pure biobased methylheptylglycerin. Tocopherol is added as a stabilizer.
- Statement of Origin
Lexgard® MHG Natural MB is derived solely from plant-based sources.
• Epichlorohydrin is derived from palm-based glycerin sourced from Thailand.
• 1-methylheptanol is derived from castor oil sourced from China.
• Tocopherol is derived from soybean (sourced from Europe and Asia) and sunflower seed (sourced from the USA).- Primary Feedstocks
- Castor
- Palm
Features & Benefits
- Benefit Claims
- Labeling Claims
- Product Features & Benefits
- Bacteriostatic booster
- Odor control benefits in deodorant applications
- Anti-soaping agent in emulsions
- Pair with silicone alternative emollient for non-soaping silicone-free emulsions
- Multifunctional medium chain glyceryl ether
- Entirely plant-based
- Effective in emulsions, creams and lotions in addition to surfactant-based products such as shower gels, facial cleansers and shampoos
- Free of parabens, formaldehyde donors, MIT, phenoxyethanol, and other traditional preservatives
- Building block for a customized Hurdle Technology system
- Micro Summary
- Controls bacteria (gram +/-) and yeast
- Use in combination with mold control agent for broad spectrum protection
- The Importance of GMPs as a Part of Hurdle Technology
Good Manufacturing Practices (GMPs) are a critical element of hurdle technology/alternative preservation!
- Clean, well-maintained facilities
- Validated cleaning and sanitization SOPs
- Micro-free purified water systems
- Micro specs and testing on all incoming raw materials Clean, protective packaging
- Validation of preservative efficacy throughout scale-up and accelerated stability testing
- Micro content testing on all manufactured lots
- Green Chemistry & Sustainable Life Cycle Highlights
Sustainable Design: Sustainability is embedded in INOLEX's design philosophy. Lexgard® MHG Natural MB design was guided by the Principles of Green Chemistry and life cycle thinking.
The following are the green chemistry & sustainable life cycle highlights:
- Renewable feedstocks
- Readily biodegradable
- Safe catalysts used
- No solvents used
- Non-aqueous product
- Benign starting materials makes for safer chemical synthesis
- Contributes to a safely preserved formula for consumer use
Applications & Uses
- Markets
- Applications
- Application Format
- Bath & Shower Applications
- Skin Care Applications
- Sun Care Applications
- Use Level
- 1.0 - 1.5% (O/W sunscreens preservation), 0.5 - 1.5% (O/W emulsion preservation), 1.0 - 1.5% (Cleansers preservation), 1.0 - 1.5% (Wipe juices preservation), 1.5 - 2.0% (Anti-soaping emulsion)
- Instruction for Use
- For use in cold or hot process
- Mix well before using
- Add to water phase (below 80°C) or during cool down phase (~40°C).
Properties
- Physical Form
- Typical Properties
- Composition
| Value | Units | Test Method / Conditions | |
| Natural Origin Index | 1.0 | — | — |
| Biobased Content | 100.0 | % | — |
| Value | Units | Test Method / Conditions | |
| Methylheptylglycerin Content | 99.9 | % | — |
| Tocopherol Content | 0.1 | % | — |
Regulatory & Compliance
- Certifications & Compliance
- Chemical Inventories
- Regulatory & Compliance Status
1223/2009 Compliance
• The product complies with Annexes III, IV, V and VI, of the Cosmetic Regulation (EC) 1223/2009.
• The product does not contain any substance listed in Annex II of the Cosmetic Regulation 1223/2009, in the limit of Article 17.
• The product complies with Article 15 ("Substances classified as CMR substances") of the Cosmetic Regulation 1223/2009.Allergen Statement: EU Fragrance
- The product does not contain any of the allergens listed in Annex II or III of Cosmetic Regulation (EC) 1223/2009.
Allergen Statement: Food
- The product does not contain tree nuts, peanuts, soybeans, wheat, eggs, milk, fish, or crustacean shellfish.
Animal Testing Statement
- INOLEX has not conducted, nor commissioned, animal testing in accordance with Regulation (EC) No. 1223/2009, Chapter V; Article 18 of the European Parliament and of the Council of 30 November 2009 on Cosmetic Products.
BSE/TSE Statement
- The product does not contain any animal derived ingredients, thus is Bovine Spongiform Encephalopathy (BSE)/Transmissible Spongiform Encephalopathy (TSE) free with respect to source, manufacture, and treatment.
California Proposition 65 Statement
- The product is not expected to contain any contaminants or by-products known to the State of California to cause cancer or reproductive toxicity as listed under Proposition 65 State Drinking Water and Toxic Enforcement Act.
CITIES Statement
- No components of the product are included on the Convention on International Trade in Endangered Species of Wild Fauna and Flora (CITES) list.
CMR Statement
- The product is not expected to contain C (Carcinogen), M (Mutagen), or R (Toxic for Reproduction) Substances as indicated on REACH Annex VI or in Regulation (EC) No. 1272/2008 (categories 1A, 1B, or 2).
Conflicts Mineral statement
Based on currently available information, INOLEX does not source raw materials from Democratic Republic of the Congo and its adjoining countries. Lexgard® Natural GC70 MB does not require the use of “Conflict Minerals” in the manufacturing process. A “Conflict Mineral” is considered to be any of the following minerals or their derivatives originating in the Democratic Republic of the Congo (the “DRC”) or any of the adjoining countries with which the DRC shares a recognized international border:
- Tin (cassiterite)
- Tantalum (columbite-tantalite)
- Tungsten (Wolframite)
- Gold
Genetic Modification Statement
- The product does not contain genetically modified material. The starting raw materials do not intentionally include genetically modified organisms (GMOs) and no GMO materials are introduced during the manufacturing process.
Gluten-Free Statement
- The product does not contain gluten. The starting raw materials do not contain gluten and no gluten is introduced during the manufacturing process.
HALAL Statement
Based on the review of the raw materials and manufacturing process, the product meets the following HALAL requirements:
• The product does not contain any ingredient of animal origin.
• The product does not contain ethyl alcohol and ethyl alcohol has not been used in the manufacturing process.
• The equipment used for manufacturing the product is not used for the manufacturing of products containing ingredients of animal origin.
• The product does not come in contact with any products of animal origin or products containing such ingredients.Heavy Metals Statement
Ag As Bi Cd Co Cr Cu <1 ppm <1 ppm <1 ppm <1 ppm <1 ppm <1 ppm <1 ppm Fe Hg Mo Pb Ni Sb Sn <1 ppm <1 ppm <1 ppm <1 ppm <1 ppm <1 ppm <1 ppm Impurites Statement
- The following list of chemicals are not expected to be present in the INOLEX Inc family of products based on our knowledge of the starting raw materials used and the current manufacturing processes – This includes strict controls of raw materials, optimized synthesis processes for the production of new chemical entities, and the use of analytical testing to ensure the quality of the finished chemicals (though not all of the below chemicals are routinely analyzed).
- Oxides (butylene oxide, ethylene oxide, propylene oxide, etc.)
- Amines (melamine, nitrosamines, etc.)
- Glycol ethers
- Silicone
- Dioxane
- Formaldehyde
- Polyaromatic Hydrocarbons
- Halogenated compounds
- Phthalates
- Parabens
- Sulfates
Please note that the above list of chemicals is not an exhaustive list
Irradiation Statement
- The product has not been subject to irradiation of any kind during or after manufacturing, and no known irradiated ingredients are present.
Kosher Statement
Based on the review of the raw materials and the manufacturing process, the product meets the following Kosher requirements:
• The product does not contain any ingredient of animal origin.
• The equipment used for manufacturing the product is not used for the manufacturing of products containing ingredients of animal origin.
• The product does not come in contact with any products of animal origin or products containing such ingredients.
• The product does not contain the following: tree nuts, peanuts, soybeans, wheat, eggs, milk, fish, and crustacean shellfish.Microbiological Testing Statement
- The product is inherently resistant to microbial contamination
Nanomaterial Statement
- Nanomaterials, in accordance with Article 16 of Cosmetic Regulation (EC) 1223/2009, are not used in the manufacturing or processing of the product and are not expected to be present.
Palm Status
- Lexgard® MHG Natural MB contains RSPO palm derivatives.
- INOLEX is a member of, and supports the principles of, the Round Table for Sustainable Palm Oil (RSPO) and RSPO efforts to encourage and certify sustainable palm oil production practices. INOLEX procures goods from suppliers that are members of the RSPO in those instances when raw materials may contain palm products.
Pesticide Statement
- The product is not expected to contain residual pesticides. The starting raw materials are sourced from pesticide-free plants, and no pesticides are introduced during the manufacturing process.
Preservatives Statement
- No preservatives are present in the product.
Residual Monomer Statement
- Not applicable.
Residual Solvent Statement
- Lexgard® MHG Natural MB conforms with both the USP <467> and ICH Q3C residual solvent guidelines. INOLEX Inc. assures that, based raw on the raw material composition, the manufacturing, handling, and storage procedures utilized at our plant sites, there is no potential for residual solvents of class 1, 2, or 3 as mentioned in the guidelines for residual solvents (CPMP/ICH/283/95) of the European Pharmacopeia, to be present in Lexgard® MHG Natural MB.
SVHC Statement
- The product does not contain any Substances of Very High Concern (SVHC) under REACH.
VEGAN Statement
- The product is suitable for vegans. It is derived from plant-based sources and does not contain any animal derived ingredients.
VOC Statement
The product does not meet the definition as a Volatile Organic Compound (VOC) under any of the following regulations:
• 40 CFR Part 51 Section 51.100
• California Air Resources Board’s definition of reactive organic gas (ROG) or total organic gasses (TOG)
• EC Directive 1999/13/EC (Solvent Emissions Directive)
• The Swiss Federal Council (based on 35a and 35c of the Environmental Protection Act)- Certifications
USDA BioPreferred® (100%)
- Regulatory Status
Glyceryl Caprylate (CAS #: 26402-26-6) EU Compliant Canada Not listed on DSL Australia Not listed on AICS Japan Compliant Korea Compliant China Not Listed on IECSC and IECIC United States Compliant New Zealand Compliant Philippines Compliant Taiwan Compliant - Standards & Certifications
- USDA Certified Biobased Content
- Readily Biodegradable
- COSMOS Approved
- NATRUE Approved
- Halal Certified
- NSF/ANSI 305 Approved
- Clean Beauty Preferred
- Kosher Compliant
- Vegan & Cruelty-free
- RSPO Mass Balanced Certified
Technical Details & Test Data
- Medium Chain Terminal Diols (MCTDs)
- MCTDs are amphiphilic compounds typically used to aid in solubilization and/or emulsification of oil-soluble species
- Their amphiphilic character enables them to interact with cell membranes (lipid bilayers), enhancing cell permeability
- Used alone or in combos, typically at 0.5 — 2.0%
- Particularly effective against bacteria
Methylheptylglycerin Lexgard™ MHG Natural MB) 100% Natural
- Science Behind
- This alternative preservation component contributes to creating a hostile environment for microbial growth as part of a Hurdle Technology approach. Lexgard® MHG Natural MB can be a complete system for certain formulations or a component of your broad spectrum customized system.
- Methylheptylglycerin is a multifunctional component. It is an anti-soaping agent and skin conditioning agent that prevents the growth of bacteria in formulation.
- Lexgard® MHG Natural MB Mechanism of Action
- Preservation Mechanism of Action: Medium-chain amphiphilic compound that interacts with cell membranes (lipid bilayers) enhancing cell permeability.
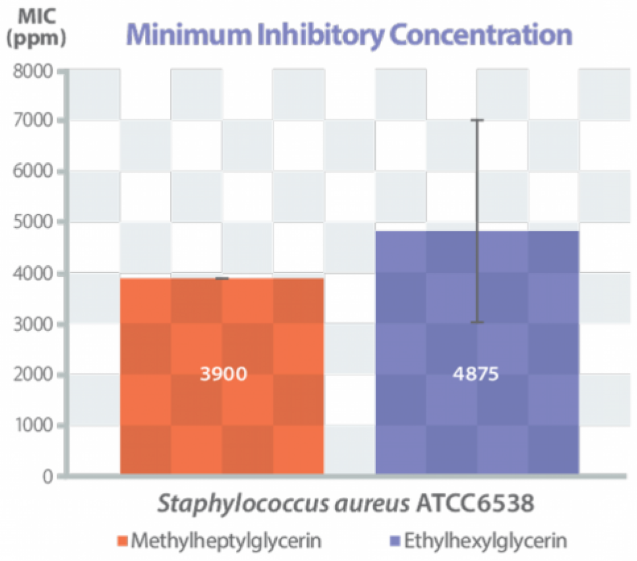
- Deodorant Odor Control Mechanism of Action: Interacts with gram+ bacteria, e.g. Staphylococcus hominis, the gram+ bacteria in the underarm area that is responsible for excreting the malodor-causing thioalcohol.
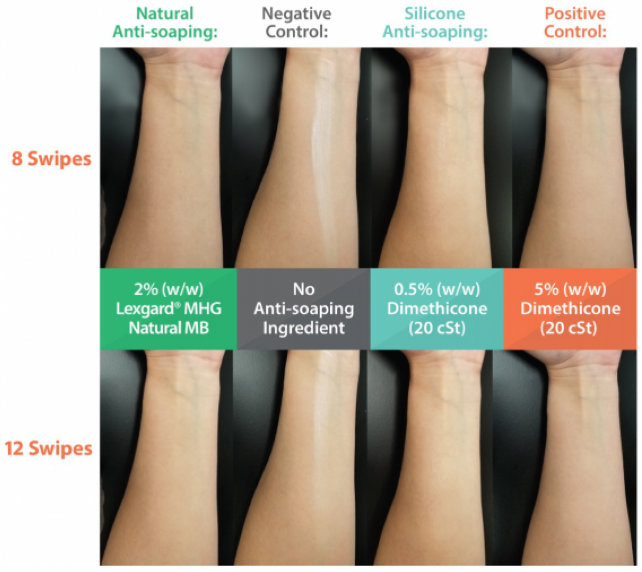
- Anti-Soaping Emulsions Mechanism of Action: Amphiphilic methyl-branched C8 carbon chain has higher dynamic surface activity than long chain emulsifiers and reduces emulsifier adsorption at the air-water interface.
- Lexgard® MHG Natural MB Test Data
Lexgard® MHG Natural MB Provides Odor Protection
Lexgard® MHG Natural MB Provides Anti-Soaping Benefit
Safety & Health
- Toxicity Review
ACUTE ORAL TOXICITY (Public Domain – REACH Registration Dossier)
Read-Across from Ethylhexylglycerin (CAS #: 70445-33-9) LD50 > 2000 mg/kg bw
ACUTE DERMAL TOXICITY (Public Domain – REACH Registration Dossier)
Read-Across from Ethylhexylglycerin (CAS #: 70445-33-9) LD50 > 2000 mg/kg bw
ACUTE INHALATION TOXICITY (Public Domain – REACH Registration Dossier)
Read-Across from Ethylhexylglycerin (CAS #: 70445-33-9) LC50 > 2.83 mg/L air
SKIN SENSITIZATION (Public Domain – CIR Scientific Literature Review)
Read-Across from Ethylhexylglycerin (CAS #: 70445-33-9) Skin sensitization was not observed in guinea pigs tested with 0.5% in theethylhexylglycerin during induction and challenged with a higher concentration (50%) maximization test (OECD 406). Local lymph node assay results for ethylhexylglycerin at concentrations up to 50% were also negative.
SKIN IRRITATION (Public Domain – REACH Registration Dossier, OECD 404)
Read-Across from Ethylhexylglycerin (CAS #: 70445-33-9)
The skin irritation potential of ethylhexylglycerin was evaluated using 3 New Zealand
White rabbits (sex not specified).21 Undiluted test substance (0.5 ml) was applied to
intact skin (shaved) and the test site was covered with a semi-occlusive dressing
secured with elastic tape. Testing was in accordance with the OECD TG 404 test
protocol. During the 4-day observation period, erythema (score = 1) was observed in
2 rabbits, but edema was not observed in any of the animals. Signs of erythema had
cleared by day 4 post-application. Ethylhexylglycerin was classified as a mild
skin irritant.EYE IRRITATION (Public Domain – REACH Registration Dossier, OECD 405)
Read-Across from Ethylhexylglycerin (CAS #: 70445-33-9)
Undiluted ethylhexylglycerin (0.1 ml) was instilled into the left conjunctival sac of
each of 3 New Zealand White rabbits (sex not specified), and animals were observed
up to day 21 post-instillation.19,21 Right eyes served as untreated controls. Testing
was in accordance with the OECD TG 405 test protocol. Conjunctival redness and
chemosis were observed in all animals and irritation scores of 2 or 3 predominated.
Conjunctival irritation persisted up to day 14, and, in one animal, corneal opacity
persisted beyond the 21-day observation period. Thus, ethylhexylglycerin caused
severe damage to the eyes of rabbits.GENOTOXICITY (Public Domain – REACH Registration Dossier – OECD 471)
Read-Across from Ethylhexylglycerin (CAS #: 70445-33-9)
Ethylhexylglycerin was found to be not mutagenic under test conditions.FRESHWATER ALGA AND CYANOBACTERIA; GROWTH INIHIBITION TEST (INOLEX Study, OECD 201)
Methylheptylglycerin (CAS #: 182015-50-5)
The calculated EC50 for percent (%) inhibition in average growth rate was 84.4 ppm for 72-hour timepoint. The calculated EC50 for percent (%) inhibition of yield was 55.9ppm for 72-hour timepoint.DETERMINATION OF BIODEGRADABILITY (INOLEX STUDY, OECD 301B)
Methylheptylglycerin (CAS #: 182015-50-5) Readily biodegradable
Storage & Handling
- Storage
Storage Conditions
- It is recommended that INOLEX, Inc. products be stored in unopened, original containers and be kept indoors in a controlled temperature environment.
Recommended Re-Evaluation Date
- Recommended re-evaluation date is 36 months from the date of the previous analysis. The recommended re-evaluation date is the time-period in which the product is expected to maintain its initial physical and chemical characteristics from the Date of Manufacture as indicated on the Certificate of Analysis. The recommended re-evaluation date period will be affected by storage conditions such as temperature, humidity, and the environment of the storage area.

