Knowde Enhanced TDS
Identification & Functionality
- Chemical Family
- Polymer Name
- Technologies
Features & Benefits
- Materials Features
- Features & Benefits
These compounds provide a unique combination of features and benefits, including:
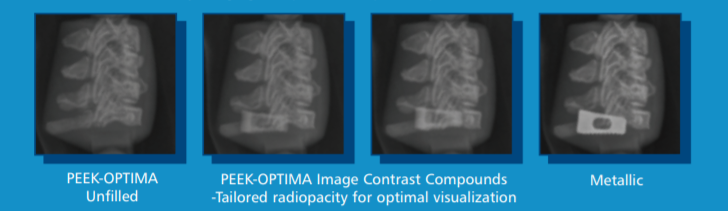
• Tailored radiopacity allows implant visibility to be optimized
• PEEK-OPTIMA polymer provides a superior combination of strength, stiffness and toughness, even after sterilization
• Biocompatibility ensures safe, long term implantation
• Processing methods allow broad design and manufacturing flexibility
• US FDA Drug and Device Master files can assist with regulatory requirements- Properties
TAILORED IMPLANT RADIOPACITY AND MEDICAL IMAGING COMPATIBILITY
Unlike metals, PEEK-OPTIMA image contrast grades provide the possibility of tailoring the visibility of an implant to suit a particular application. It is therefore possible to achieve an appropriate balance of implant, bone and tissue visualization without arti_x0002_facts or scatter.
DESIGN AND PROCESSING FLEXIBILITY
Available in a range of viscosities (standard, medium and low), PEEK-OPTIMA image contrast grades can be processed by conventional methods, including injection molding and extrusion, and can be machined, allowing medical device manufacturers broad design and manufacturing flexibility.
PROVEN BIOCOMPATIBILITY Extensive testing of PEEK-OPTIMA compounds to ISO 10993 standards demonstrated no evidence of cyto_x0002_toxicity, systemic toxicity or irritation. Results have been lodged with the US FDA and can reduce the time and expense of the approval process.
Applications & Uses
- Markets
- Plastics & Elastomers End Uses
- Plastics & Elastomers Processing Methods
Properties
- Typical Properties
| Value | Units | Test Method / Conditions | |
| Tensile Strength | 95 | MPa | ISO 527 |
| Tensile Elongation (Break) | 20 | % | ISO 527 |
| Flexural Modulus | 3.8 | GPa | ISO 178 |
| Flexural Strength | 150 | MPa | ISO 178 |
| Notvhed Izod Impact | 7 | kJ/m | ISO 180 |
| Specific Gravity | 1.36 | g/cc | ISO 1183 |
Regulatory & Compliance
- Certifications & Compliance
- Quality & Integrity Assured
In compliance with ISO 13485:2003 and ISO 9001:2000 standards, Invibio embraces all the principles of Good Manufacturing Practice (GMP) in relation to the manufacture of PEEK-OPTIMA image contrast compounds
Technical Details & Test Data
- CT Imaging Comparison of Spinal Implants in a Phantom
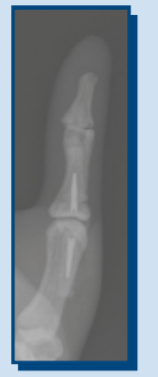
- X-ray Imaging Comparison of Spinal Implants in a Phantom