Knowde Enhanced TDS
Identification & Functionality
- Ingredient Name
- Food Ingredients Functions
- Pharma & Nutraceuticals Functions
- Ingredients
- Bifidobacterium Lactis
Features & Benefits
- Benefit Claims (Health)
- Food Ingredients Features
- Product Highlights
- Researched in numerous human clinical studies with subjects ranging from the elderly, children, and infants.
- A stable probiotic manufactured to reduce die off rate.
- Can be formulated into supplements, yogurt, powdered beverages, confectionaries, and high fiber juices.
- Benefits of BifidoB HN019™
- Promotes bowel movement by decreasing the intestinal transit time
- Improves protection against diarrhea inducing pathogens
- May reduce infection induced inflammation
- May improve the availability of iron in the body
Applications & Uses
- Markets
- Applications
- Food & Nutrition Applications
Regulatory & Compliance
Technical Details & Test Data
- Clinical Study of BifidoB HN019™
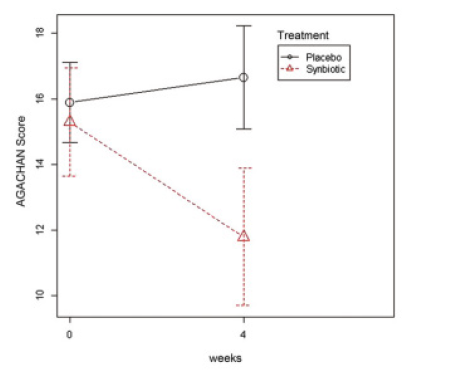
Average and confidence interval of the AGACHAN score of constipated women self-reported at the basal week and after 4-weeks treatment with synbiotic (n-24) or placebo (n-26). Nonparametric ANOVA for repeated measures and Wilcoxon.

