Knowde Enhanced TDS
Identification & Functionality
- Ingredient Name
- Food Ingredients Functions
- Ingredients
- Water, Calcium Sulfate
- Food Additive Number
- E 516, INS 516
- Technologies
Features & Benefits
- Food Ingredients Features
- Why should I use FreshpHix in my plant?
- The key to effective chlorine use is to keep it in the form of HOCl (hypochlorous acid). This is accomplished by controlling the pH of the water.
- Managing the pH and effectively utilizing the chlorine can be accomplished cost-effectively through the use of FreshpHix*.
*Amount of FreshpHix required to adjust water pH may vary by location depending upon water quality (pH and dissolved chemicals such as minerals, bicarbonates, etc.)
- Features & Benefits
- It is effective in all water temperatures.
- No fumes or odors.
- Highly concentrated so the dilution ration is very high.
- Economical to use.
- Self-adjusting system to maintain ph / ORP.
Applications & Uses
- Markets
Properties
- Physical Form
Regulatory & Compliance
- Certifications & Compliance
Technical Details & Test Data
- The Science and Necessity of pH Control
- To accomplish disinfection, a chemical reaction between chlorine and water (hydrolysis) must occur to form hypochlorous acid (HOCI).
- Regardless of the chlorine source, the desired end product is hypochlorous acid (HOCI). This is the chemical agent that destroys the bacteria and other potential contaminants.
- Hypochlorous acid is a "weak" acid, which means that it tends to undergo partial dissociation as follows:
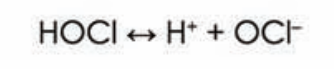
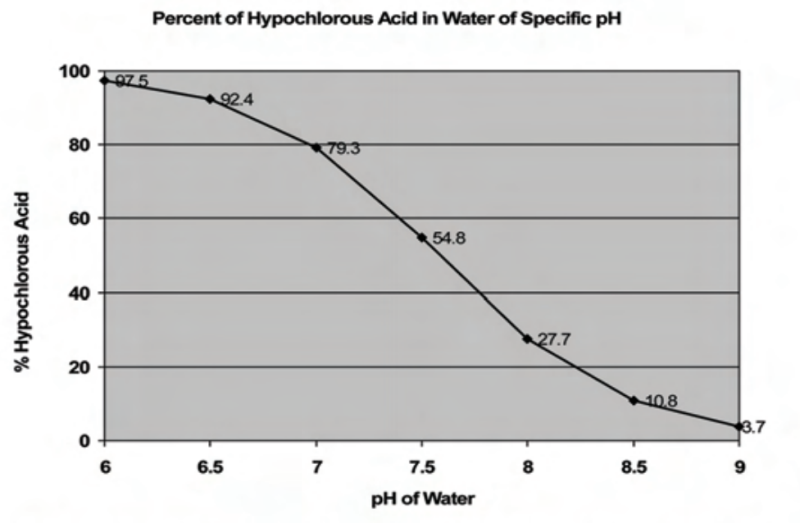
As seen in the chart below, approximately 28% of the free available chlorine (FAC) exists in the HOCI form in water with a pH of 8.0, whereas, approximately 92% of the FAC exists as HOCI at a pH of 6.5. Thus, it would require approximately 3.25 times more bleach in water with pH 6.5 to achieve the same results in water with a pH of 8.0.
The germicidal effects derived from the chlorination of water are well documented. However, scientific principles must be followed for the chlorine to be effective. The hit-or-miss approach will not be effective. It cannot be over emphasized that simply "hooking up chlorine and letting it run" will not accomplish the antimicrobial or germicidal results that are desired. Moreover. this approach may produce undesirable effects. e.g. noxious odors and an unhealthy work place environment. Managing the pH and effectively using the chlorine is key, which can be accomplished cost-effectively through the use of FreshpHix.

