Knowde Enhanced TDS
Identification & Functionality
- Plastics & Elastomers Functions
- Technologies
- Product Families
Features & Benefits
- Labeling Claims
- Materials Features
- Product Highlights
xMED412 is an energy-curable resin used to manufacture a variety of 3D printed biocompatible medical devices. Due to the physical properties and biocompatibility of the finished material, 3D printed parts can be used in a variety applications when processed in accordance with validated workflows. If sterile parts are required, please follow the guidance in this IFU to obtain both a safe and effective final device.
xMED412 parts have been cleared through clinical studies for the use of Nasopharyngeal Swabs. Parts are processed in the recommended methods below. Sterile nasopharyngeal swabs manufactured with Nexa3D xMED412 are in compliance with ISO 10993 testing and FDA Class I Exempt classification.
- Additional Development Options
Colors : xMED412 formula is made with additional pigment colors.
Formula Modification xMED412 has potential for tensile property adjustments.
Applications & Uses
- Markets
- Plastics & Elastomers End Uses
- Plastics & Elastomers Processing Methods
- Post Processing
xMED412 requires post processing to achieve specified properties. Prior to post curing, support structures should be removed from the printed part, and the part should be washed in a compatible cleaner. Nexa3d recommends either IPA or Cleaner C™ in 2 minute interval wash cycles. Use compressed air to remove residual solvent from the surface of the material between intervals.
- Post Curing
xMED412 requires post curing to achieve specified properties. A wide array of post cure equipment can be used to cure appropriately.
- Directions for Use
- Nest the parts you would like to print in a CAM software. For swab parts, nest them in a vertical orientation and packed to maximize the build area without compromising print quality.
- Prior to use, agitate the bottle of resin and allow the resin to adjust to an ambient temperature between 20-25°C / 68-77°F.
- Only print Nexa3D xMED412 on NXE400 with settings validated by Nexa3D. Alternative printers must be validated by Nexa3d to determine print settings needed to generate a safe and effective device.
- Limitations
Post Cure : xMED412 requires a UV/ Visible light post cure.
- Directions for Post-Processing
- When the print is complete, gently remove parts from the printer build platform with a scraper or blade.
- Wash the parts for the pre-determined duration and number of wash cycles Henkel has validated for the workflow you will be using.
- Dry the parts with compressed air and inspect parts for any residual resin, which will have a glossy appearance.If any residual resin is observed, repeat step 2.
- Allow the parts to rest at room temperature for 30-90 minutes beforeprogressing.
- Place parts in a single layer in a post-cure unit Henkel has validated and use the post-cure unit specific settings.
- Alternative post-cure units must by validated by Henkel to determine post-cure settings to generate a safe and effective device.
- Directions for Sterilization
Autoclave Steam Sterilization : 134°C (273°F)
- Samples should be packaged and distributed in the appropriate sterilization bags.
- Bags should be laid flat and not stacked on top of one another to ensure adequate steam saturation.
- Ramp temperature up to 134°C (273°F) and pressurize to 2.1 bar (30.5psi).
- Hold for 4 minutes.
- Depressurize chamber to -1.0 bar (-14.5 psi) and hold for a minimum of 13 minutes. Temperatures may vary during the depressurization. If the temperature stays above 60°C in the depressurization phase, the finished parts will be in compliance.
- Check that the bag sterilization confirmation marks are fulfilled. Inspect parts for clarity. If parts are cloudy or opaque, parts were not dried completely and should not be used.
Autoclave Steam Sterilization : 121°C (250°F) Short
- Samples should be packaged and distributed in the appropriate sterilization bags.
- Bags should be laid flat and not stacked on top of one another to ensure adequate steam saturation.
- Ramp temperature up to 121°C (250°F) and pressurize to 1.1 bar (16.0psi). Hold for 20 minutes.
- Depressurize chamber from -0.7 (10.1 psi) to -1.0 bar (-14.5 psi) and hold for 5 minutes. Temperatures may vary during the depressurization. If the temperature stays above 60°C in the depressurization phase, the finished parts will be in compliance.
- Check that the bag sterilization confirmation marks are fulfilled.
- Inspect parts for clarity. If parts are cloudy or opaque, parts were not dried completely and should not be used.
Autoclave Steam Sterilization: Extended 121°C (250°F) Custom
- Samples should be packaged and distributed in the appropriate sterilization bags.
- Bags should be laid flat and not stacked on top of one another to ensure adequate steam saturation.
- Ramp temperature up to 121°C (250°F) and pressurize to 1.1 bar (16.0psi). Hold for 30 minutes.
- Depressurize chamber from -0.7 (10.1 psi) to -1.0 bar (-14.5 psi) and hold for 60 minutes. Hold temperature at 97°C (207°F) during drying phase.
- Check that the bag sterilization confirmation marks are fulfilled.
- Inspect parts for clarity. If parts are cloudy or opaque, parts were not dried completely and should not be used.
Properties
- Color
- Mechanical Properties
- Typical Properties in Liquid State
- Note
All specimen are printed unless otherwise noted. All specimen were conditioned in ambient lab conditions at 19-23C / 40-60% RH for at least 24 hours. ASTM Methods: D638 Type IV, 5mm/min, D790-B, 2mm/min, D256 Notched IZOD (Machine Notched), 6 mm x 12 mm, D648, D2240, Type “D” (0, 3 seconds), D570 0.125” x 2” Disk 24hr@ 25°C, D1475, D7867@ 25°C (77°F)
1. TaskID Reference: FOR17060
2.TaskID Reference: FOR17061
3.TaskID Reference: FOR17059
4. TaskID Reference: FOR20031
5. TaskID Reference: FOR17058
6.TaskID Reference: FOR17057
7.TaskID Reference: FOR17470
8. TaskID Reference: FOR17471
9. TaskID Reference: FOR17473
10.TaskID Reference: FOR17479
11.TaskID Reference: FOR17478
12.TaskID Reference: FOR17689- Color Properties
Ultra Clear Color Properties
Method: ASTM E308, Total Transmission Part State L* a* b* C* h dE Green / no post-processing [17] 94.61 -1.4 2.23 2.64 122.17 NA Dymax 5000EC 10 minutes / side [17] 94.16 -0.46 0.76 0.89 121.33 1.801943 Loctite CL36 30min/side [18] 93.77 -0.5 1.01 1.13 116.3 1.733205 QUV exterior weathering conditions (ASTM G-154-Cycle 1) : Ultra Clear Color
Method: ASTM G-154—Cycle 1 & ASTM E308, Total Transmission QUV Exposure Time (Hrs) L* a* b* C* h dE 0 93.49 -0.63 1.1 1.27 119.63 NA 325 92.03 -0.66 2.45 2.54 105.14 1.988718 650 91.69 -0.8 3.46 3.56 102.96 2.972961 QUV exterior weathering conditions (ASTM G-154-Cycle 1) : Ultra Clear Color Mechanical Properties
Method: ASTM G-154—Cycle 1 QUV Exposure Time (Hrs) Tensile Stress at break (MPa) Yield Stress (MPa) Young’s Modulus (MPa) Elongation at break (%)0 38 ± 1.4 29.4 ± 1.3 1245 ± 43 141 ± 4 24 36.0 ± 4 26.0 ± 1.9 1170 ± 84 145 ± 15 192 32.0 ± 3 23.0 ± 0.3 1025 ± 15 142 ± 17 325 28.1 ± 4 32.9 ± 0.64 1394 ± 32.85 80.8 ± 28.78 650 27 ± 0.84 26.5 ± 0.68 1297 ± 27.77 105 ± 6.357
| Value | Units | Test Method / Conditions | |
| Tensile Strength at Break (Green)¹² | 19 - 21.6 | MPa | ASTM D638 |
| Tensile Stress at Yield (Green)¹² | 16 - 21 | MPa | ASTM D638 |
| Young’s Modulus (Green)¹² | 691 - 903 | MPa | ASTM D638 |
| Elongation at Failure (Green)¹² | 142.5 - 155.5 | % | ASTM D638 |
| Solid Density (Green)¹⁶ | 1.129 | g/cm³ | ASTM D1475 |
| Tensile Strength at Break (Post Processed)¹ | 36.6 - 39.4 | MPa | ASTM D638 |
| Tensile Stress at Yield (Post Processed)¹ | 28.06 - 30.66 | MPa | ASTM D638 |
| Young’s Modulus (Post Processed)¹ | 1202 - 1288 | MPa | ASTM D638 |
| Elongation at Failure (Post Processed)¹ | 137 - 145 | % | ASTM D638 |
| Flexural Stress at Yield (Post Processed)² | 35.04 - 40.16 | MPa | ASTM D790 |
| Flexural Modulus (Post Processed)² | 946 - 1098 | MPa | ASTM D790 |
| Flexural Strain at Break (Post Processed)² | min. 10 | % | ASTM D790 |
| IZOD Impact Strength (Notched, Post Processed)³ | 37.6 - 47.6 | J/m | ASTM D256 |
| HDT (at 0.455 MPa, Post Processed)¹⁵ | 40 | °C | ASTM D648 |
| Hardness (0s, Post Processed)⁴ | 74 | Shore D | ASTM D2240 |
| Water Absorption (24 hr, Post Processed)⁵ | 0.36 | % | ASTM D570 |
| Ec (Post Processed)¹⁴ | 7.81 | mJ/cm² | Internal |
| Dp (Post Processed)¹⁴ | 0.166 | mm | Internal |
| Solid Density (Post Processed)¹⁶ | 1.146 | — | ASTM D1475 |
| Hardness (3s, Post Processed)⁴ | 70 | Shore D | ASTM D2240 |
| Value | Units | Test Method / Conditions | |
| Viscosity (25°C)⁶ | 487 - 787 | cP | — |
| Liquid Density¹⁶ | 1.0614 | — | ASTM D1475 |
Regulatory & Compliance
- Certifications & Compliance
- Chemical Inventories
Technical Details & Test Data
- Physical Properties of Sterilized Parts
ASTM D638 Type V dog bones and NP swabs were printed with Nexa3D xMED412 and sterilized with autoclave steam, EtO, and gas plasma sterilization processes. Each sterilization method had a sample set of n=6. Test samples were compared to a control sample set of n=3.
Autoclave Steam Sterilization : 121°C (250°F) Short
The tensile results of 121°C sterilized dog bones parts showed that the Young’s Modulus, Maximum Tensile Strength, and % Strain at break all displayed values within the standard deviation of the non-sterilized control samples. This result indicates little to no physical property effects when subjected to the described sterilization procedure/conditions.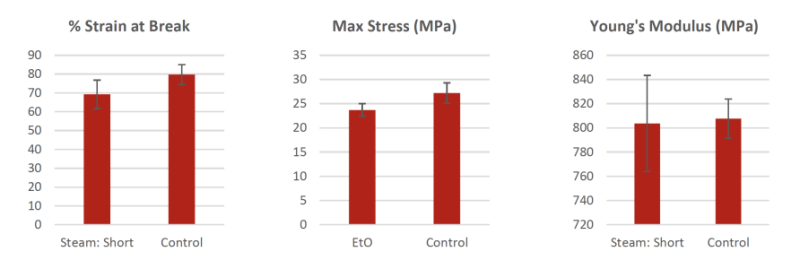
Autoclave Steam Sterilization : Extended 121°C (250°F) Custom
The tensile results of EXTENDED Cycle 121°C Custom sterilized dog bones parts showed that the % Strain at break all displayed values within the standard deviation of the non-sterilized control samples. The Young’s Modulus and Maximum Tensile Strength show values that are higher than the control samples. Because the % Strain at break is within the range of the control samples, these higher values are not deemed to be a symptom of material degradation or any negative performance change.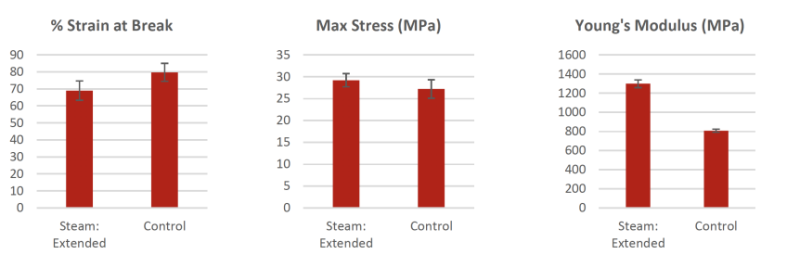
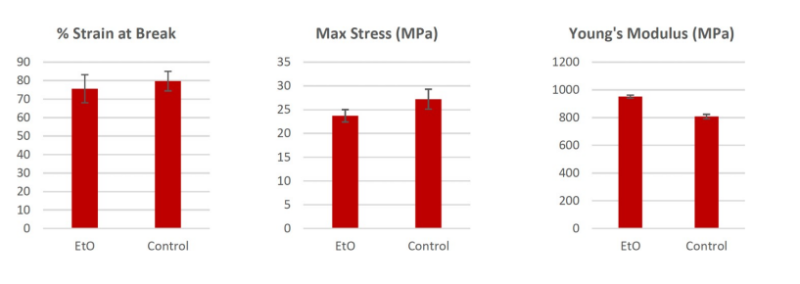
Ethylene Oxide (EtO) Sterilization
The tensile results of the Ethylene Oxide sterilized dog bone parts showed that the Young’s Modulus, Maximum Tensile Strength, and % Strain at break all displayed values within the standard deviation of the 121°C Solid/Unwrapped and the non-sterilized control samples. This result indicates little to no physical property effects when subjected to the described sterilization procedure/conditions. These properties are an effective property set for nasopharyngeal swabs.
- Biocompatibility of Sterilized Parts
ISO 10993-5 Cytotoxicity
Parts were prepared and sterilized in accordance with this document and submitted to Pacific BioLabs for evaluation in accordance with ISO 10993-5, Biological evaluation of medical devices - Part 5: Tests for in vitro cytotoxicity. Each sterilization method had a sample set of n=4.
Test samples were compared to a control sample set of n=4. Passing scores according to ISO10993-5 were observed from the control, steam autoclave (121°C Solid/Unwrapped program), and ethylene oxide sterilization methods.
Sterilization Method 24-Hour Exposure 48-Hour Exposure
EtO 0 0 Steam: Short 0 0 Control 0 0
Storage & Handling
- Warnings and Precautions
- Parts must be printed and post processed in accordance with approved workflows prior to use.
- Keep finished parts stored in a cool, dry place (15-30°C) and away from sunlight.
- Finished parts are not meant to be used for prolonged periods in outdoor environments.
- The Nexa3D xMED412 material contains (meth)acrylate monomers and oligomers which, although rare, may cause an allergic reaction in individuals sensitive to acrylic containing products.

