Knowde Enhanced TDS
Identification & Functionality
- Ingredient Name
- Pharma & Nutraceuticals Functions
- Technologies
- Product Families
- Natural Source
Injuv® is derived from rooster combs, which have been safely used as a source of hyaluronic acid for more than 20 years, in the form of an injectable treatment for osteoarthritis of the knee. Not only does this type of hyaluronic acid have a natural affinity for human tissues, it has been clinically shown not to cause an allergic response.
Features & Benefits
- Labeling Claims
- Benefits
In a pre-clinical trial conducted at Otsuma Women’s University in Japan, 96 women ages 22 to 65 took Injuv® (six 70-mg tablets per day) for 45 days. Subjective interview questionnaires revealed dramatic improvements in skin moisture, smoothness, and softness.10
• 84% reported a great improvement in the moisture
levels of their hands and face
• 83% reported a great improvement in the smoothness
of their skin
• 78% reported a great improvement in the softness
of their elbows, knees, and hands
• 50% reported a great improvement in the stiffness
of their jointsAnimal Study Indicates Injuv®
Aids Wound Healing
A study in mice found that oral administrationof Injuv® (3000 mg/kg) significantly improvedwound healing compared to placebo (saline) afterjust three days.11Toxicity Test Demonstrates
Safety of Injuv®
In an acute toxicity test, rats were administered a very high single dosage of Injuv®. No observable abnormalities were observed and no mortalities occurred. The acute oral LD50 of Injuv® was determined to be greater than 5,050 mg/kg — equivalent to 343,400 mg for the average human.12
Applications & Uses
- Markets
- Delivered to the Skin
Of course, it doesn’t matter if something is absorbed if it’s not delivered where it’s needed in the body. The absorption and delivery of Injuv® has been demonstrated using a well-established animal model to measure wound healing capacity. The physiological activity of Injuv® — illustrated by the fact that rats treated with oral Injuv® experienced significantly improved wound healing compared to those given placebo — is a reliable indicator that Injuv® is absorbed and delivered to the target tissue: the skin.
Technical Details & Test Data
- Injuv® Original Research
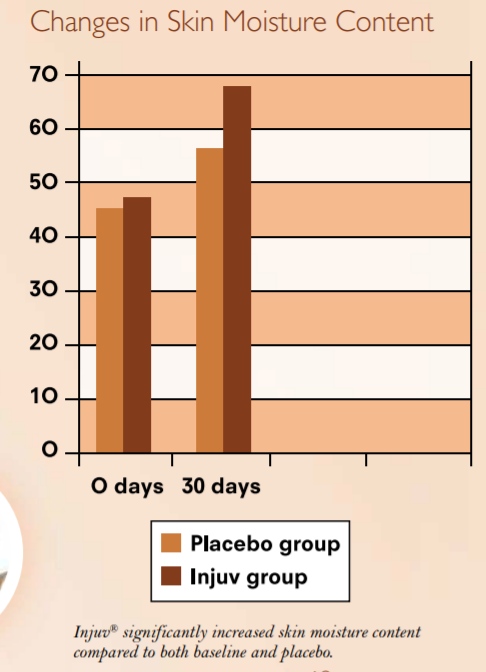
Clinical Study Finds Injuv® Improves
Skin Moisture Content
A placebo-controlled human clinical study was undertaken to determine whether Injuv® could improve the moisture content of the skin. A total of 107 volunteers aged 30 to 50 years, with an average age of 45, were randomly assigned to take either
Injuv® (two 70-mg soft gels twice daily) or placebo for 30 days.
Skin surface moisture content was measured before and after supplementation using an SHP88 probe.
Subjects who took Injuv® showed a statistically significant increase in skin moisture content compared to both baseline and placebo. There were no adverse events reported.