Knowde Enhanced TDS
Identification & Functionality
- Chemical Name
- Molecular formula
- C₈H₁₀N₄O₂ · H₂O
- Technologies
- Product Families
- Definition
Caffeine is anhydrous or contains one molecule of water of hydration. It contains not less than 98.5% and not more than 101.0% of C₈H₁₀N₄O₂, calculated on the anhydrous basis.
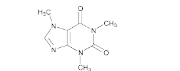
- Chemical Structure
Applications & Uses
- Markets
Properties
- Physical Form
- Solubility
- Appearance
- White crystalline powder
- Typical Properties
| Value | Units | Test Method / Conditions | |
| Melting Point | 236.6 | °C | — |
| Molecular Weight | 194.19 | — | — |
| Acidity (0.01 mol/L NaOH) | max. 0.2 | ml | — |
| Organic Impurities (individual impurity) | 0.02 | % | — |
| Total Impurities | 0.03 | % | — |
| Sulfate Content | max. 500 | ppm | — |
| Heavy Metals (as Lead) | max. 0.001 | % | — |
| Loss on Drying | 0.05 | % | — |
| Residue on Ignition | 0.01 | % | — |
| Sulphated Ash Content | 0.01 | % | — |
| Assay Content (on dried basis) | 100. 1 | % | — |
| Assay Content (on dried basis) | 100.0 | % | HPLC |
| Particle Size (Through 80 mesh) | 90.2 | % | — |
Regulatory & Compliance
- Certifications & Compliance
- Chemical Inventories
- USP Reference Standards
- USP Caffeine RS
Packaging & Availability
- Labelling Information
Label it to indicate whether it is anhydrous or hydrous.
