Knowde Enhanced TDS
Identification & Functionality
- Chemical Name
- Pharma & Nutraceuticals Functions
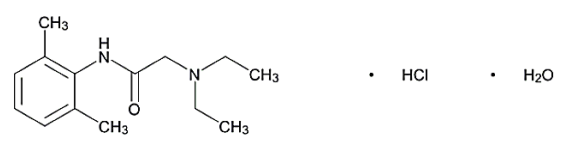
- Molecular formula
- C₁₄H₂₂N₂O · HCl · H₂O
- Technologies
- Product Families
- Definition
Lidocaine Hydrochloride contains not less than 97.5% and not more than 102.5% of lidocaine hydrochloride (C₁₄H₂₂N₂O · HCl), calculated on the anhydrous basis.
- Chemical Structure
Applications & Uses
- Markets
Properties
- Physical Form
- Typical Properties
| Value | Units | Test Method / Conditions | |
| Molecular Weight | 288.81 | — | — |
Regulatory & Compliance
- Certifications & Compliance
- Chemical Inventories
- USP Reference Standards
- USP Lidocaine Hydrochloride RS USP Lidocaine Related Compound H RS 2-Chloro-N-(2,6-dimethylphenyl)acetamide
- C10H12ClNO 197.66 USP Ropivacaine Related Compound A RS 2,6-Dimethylaniline hydrochloride
- C8H11N · HCl 157.64
Packaging & Availability
- Labelling Information
Where it is intended for use in preparing injectable dosage forms, the label states that it is sterile or must be subjected to further processing during the preparation of injectable dosage forms.
Storage & Handling
- Storage Information
Preserve in well-closed containers. Store at controlled room temperature.
