Knowde Enhanced TDS
Identification & Functionality
- Active Component
- Ingredient Name
- Ingredients
- Crocus Sativus L.
Features & Benefits
- Benefit Claims (Health)
- Labeling Claims
- Food Ingredients Features
- Product Features
- Standardized to ≥3.5% Lepticrosalides® by HPLC-DAD.
- Well-tolerated: No side effects have been reported in 740 participants
- High stability.
- Halal and Kosher certified
- Proven bioavailability (pharmacokinetics study).
- Rapid absorption in 1 hour.
- Lowest dosage: 28 mg/day.
- Benefits of affron®
- USA Award-winner Ingredient for Cognitive Health 2020.
- 8 clinical studies published*.
- Proprietary extraction process AFFRON Cool-Tech, which uniquely concentrates and preserves the bioactives with lower energy use.
- Genuine Crocus sativus L. Made in Spain.
- Own extraction plant.
- Lowest dosage: 28 mg/day.
- Unique dose-response study in 128 healthy adults.
- Clinically studied in adolescents.
- Product Highlight
Affron® improved mood on menopausal women
A new, randomized, double-blind, placebo-controlled study showed that perimenopausal women taking affron® for 12 weeks experienced a reduction of 32-33% in low mood and occasional stress, without undesirable estrogenic effects.
The Fourth Frontier: Performance and sleep with affron®
Sleep has never been more relevant to sports nutrition and growth active nutrition.
The results demonstrate that affron® is efficacious at improving restorative sleep-in individuals with self-reported sleep disturbance. On the basis that sport, and exercise, increases the risk of sleep disturbance, there is good rationale to use affron® in sports nutrition.
Affron® offers a proven, natural, and plant-based solution that is versatile to use in multiple formats and formulations.Affron® improved sleep quality at a low dose
A new, randomized, double-blind, placebo-controlled study provided further confirmation of the sleep-enhancing effects of 28 days of affron® supplementation at 14 mg, 1 hour before bedtime, facilitating consumer’s adherence.
By the very first time, a single dose of affron® 1 hour before sleep revealed the new possible mechanism of action influencing sleep hormones.Best Practices Award 2020
We have been recognized by Frost & Sullivan, for Helping People Improve their Cognitive Functions with our Saffron-based Ingredient, Affron®.
Affron® has been recognized as the Ingredient of the Year for Cognitive Function, at The NutraIngredients USA Awards, 2020.
Affron® is the most published saffron extract in the world, reaching 6M of potential audience
1 million people worldwide benefit from affron® to maintain a positive mood and mental balance every day- Maintain a positive mood
- Induce relaxation, improve occasional stress, anxiety, and tension
- Sleep better to address occasional sleeplessness
Applications & Uses
- Markets
- Use Level
- 28 mg/day
- Mechanisms of Action
The following main mechanisms of action of affron® have been identified:
- Inhibition of the reuptake mechanism of dopamine, norepinephrine, and serotonin, increasing the concentrations of these neurotransmitters in the synaptic cleft of neurons, thereby improving mood balance.
- Antioxidant effect against the excessive oxidative stress produced during occasional anxiety or stress processes in the central nervous system.
- Anti-inflammatory action in stressed neural tissue, decreasing hippocampal levels of apoptotic biomarkers and stress signal molecules.
Properties
- Solubility
- Soluble in
- Water
Regulatory & Compliance
Technical Details & Test Data
- Improved mood and Evidence in Adolescents
1st saffron extract safe and effective to use in combination with antidepressants
In a randomized double-blind, placebo-controlled study (N=68), the administration of affron® for 8 weeks was associated with reduced side effects associated with antidepressant intake and increased antidepressant effects in adults currently taking pharmaceutical antidepressants.
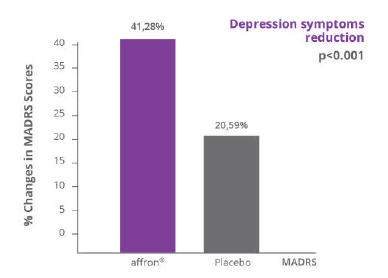
Affron® improved mood on menopausal women
In a randomized, double-blind, placebo-controlled study (N=86) affron® intake of 28mg/day for 12 weeks, significantly improved the psychological aspects of the Greene Climacteric Scale (GCS), characterized by
- 33% reduction in anxiety symptoms
- 32% reduction in depression symptoms
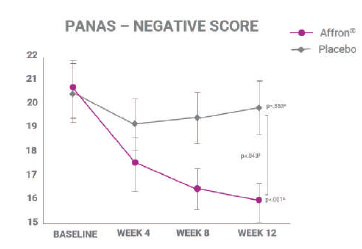
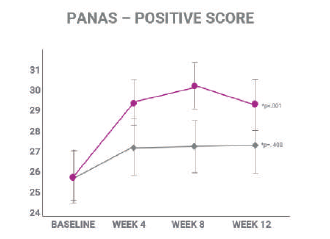
There was a significant reduction of 22% in the PANAS negative aspects score. Moreover, affron® show a strong tendecy to improve positive aspects.
Performance, recovery and sleep
- Recovery aims to restore physiological and psychological processes to achieve the optimum level.
- Two of the most popular interventions are nutrition and sleep.
- Sleep is the fourth stage of performance. To prepare, perform, recover, and sleep.
Affron® improved enjoyment in active adults
In this 6-week, randomized, double-blind, placebo-controlled trial, supplementation with 14 mg, twice daily of a saffron extract (affron®) in recreationally active adults was associated with improvements in self-reported exercise enjoyment and mood.
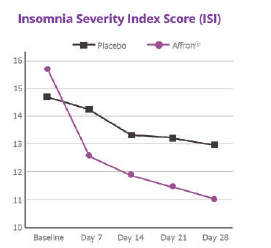
Affron® improves occasional insomnia
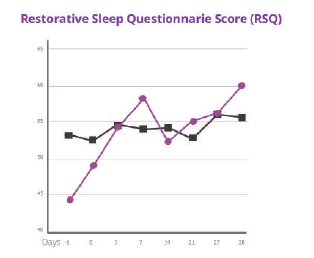
Affron® intake for 28 days was associated with greater improvements in:- Sleep quality as measured by the ISI, with most changes occurring in the first 7 days of treatment.
- Restorative sleep as measured by the RSQ.
- Sleep quality and strong trends suggesting greater reductions in the number of awakenings after sleep onset and increases in alertness upon awakening.
Satisfaction ratings indicated that 96% of participants in the affron® group were satisfied with their tablet intake.
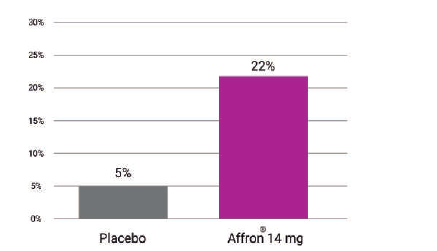
Affron® improved sleep quality
In a three-arm, parallel-group, randomized, double-blind, placebo-controlled study (N=120) affron® intake of 14mg/day one hour before sleep, validated and extended on previous positive findings of the sleep-enhancing effects of affron® in adults with unsatisfactory sleeps. "There was an approximately 25% reduction in insomnia at the end of the study".
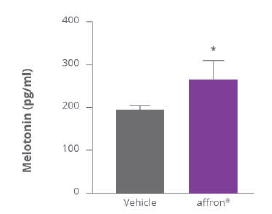
Affron® increased melatonin concentration
Melatonin concentration levels increased in concentration from baseline to 4-weeks compared to placebo (p=0.036) with a single dose one hour before sleep®
Change in evening Melatonin (pg/ml)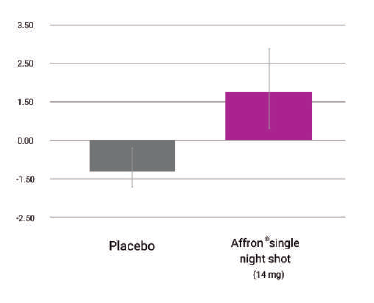
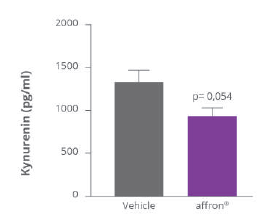
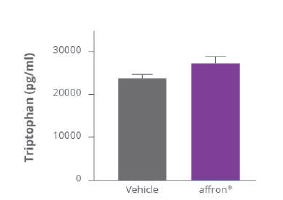
Affron® stimulates endogenous melatonin production
The reduction of kynurenine levels is explained by the fact that affron" acts by inhibiting key enzymes in the transformation of tryptophan into kynurenine, Affron® intake significantly enhances the presence of tryptophan available for the melatonin metabolic pathway.
- Technical Data
1st saffron extract safe and effective to use in combination with antidepressants
In a randomized double-blind, placebo-controlled study (N=68), the administration of affron® for 8 weeks was associated with reduced side effects associated with antidepressant intake and increased antidepressant effects in adults currently taking pharmaceutical antidepressants.

Affron® improved mood on menopausal women
In a randomized, double-blind, placebo-controlled study (N=86) affron® intake of 28mg/day for 12 weeks, significantly improved the psychological aspects of the Greene Climacteric Scale (GCS), characterized by
- 33% reduction in anxiety symptoms
- 32% reduction in depression symptoms
There was a significant reduction of 22% in the PANAS negative aspects score. Moreover, affron® show a strong tendecy to improve positive aspects.


Performance, recovery and sleep
- Recovery aims to restore physiological and psychological processes to achieve the optimum level.
- Two of the most popular interventions are nutrition and sleep.
- Sleep is the fourth stage of performance. To prepare, perform, recover, and sleep.
Affron® improved enjoyment in active adults
In this 6-week, randomized, double-blind, placebo-controlled trial, supplementation with 14 mg, twice daily of a saffron extract (affron®) in recreationally active adults was associated with improvements in self-reported exercise enjoyment and mood.
Affron® improves occasional insomnia
Affron® intake for 28 days was associated with greater improvements in:- Sleep quality as measured by the ISI, with most changes occurring in the first 7 days of treatment.
- Restorative sleep as measured by the RSQ.
- Sleep quality and strong trends suggesting greater reductions in the number of awakenings after sleep onset and increases in alertness upon awakening.
Satisfaction ratings indicated that 96% of participants in the affron® group were satisfied with their tablet intake.


Affron® improved sleep quality
In a three-arm, parallel-group, randomised, double-blind, placebo-controlled study (N=120) affron® intake of 14mg/day one hour before sleep, validated and extended on previous positive findings of the sleep-enhancing effects of affron® in adults with unsatisfactory sleeps. "There was an approximately 25% reduction in insomnia at the end of the study".

Affron® increased melatonin concentration
Melatonin concentration levels increased in concentration from baseline to 4-weeks compared to placebo (p=0.036) with a single dose one hour before sleep®
Change in evening Melatonin (pg/ml)
Affron® stimulates endogenous melatonin production
The reduction of kynurenine levels is explained by the fact that affron" acts by inhibiting key enzymes in the transformation of tryptophan into kynurenine, Affron® intake significantly enhances the presence of tryptophan available for the melatonin metabolic pathway.



- Proprietary extraction process: AFFRON Cool-Tech
It is a patented, low-temperature production process that creates highly concentrated affron® with long-lasting actives’s stability of at least 36 months. This technique allows us to get superior saffron Quality with less industrial processing, less energy use and zero chemicals.
Storage & Handling
- Shelf Life
- 3 years

