Knowde Enhanced TDS
Identification & Functionality
- Ingredient Name
- Food Ingredients Functions
- Pharma & Nutraceuticals Functions
- Molecular formula
- C7H4NO3SNa.2H2O
- CAS No.
- 6155-57-3
- Ingredients
- Sodium Saccharin
- Food Additive Number
- E 954(iv), INS 954(iv)
- Product Families
- Additive Products
Supplies sodium saccharin mainly to the high-end of the food market and to the pharmaceutical industry.
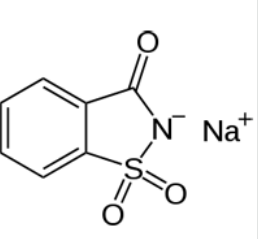
- Chemical Structure
Features & Benefits
- Labeling Claims
- Food Ingredients Features
Applications & Uses
- Applications
- Food & Nutrition Applications
- Main Applications
Excipient for pharmaceutical products, toothpaste, mouthwash, beverages, table-top, sweeteners, confectionery, electro-plating.
Properties
- Solubility
- Appearance
- White or almost white, odourless micro crystals.
- Taste
- Intensely sweet
- Soluble in
- Freely Water
- Sparingly soluble in
- Ethanol
- Microbiological Values
- Specifications
| Value | Units | Test Method / Conditions | |
| Total Aerobic Mesophilic Bacteria | max. 1000 | cfu/g | Eur. Ph. |
| Total Coliform Bacteria | Absence / 0.1 | g | - |
| Salmonella Spp. | Absence/25 | g | - |
| Mould And Yeast | max. 100 | - | - |
| Value | Units | Test Method / Conditions | |
| Ph. Eur. Monograph | Nr 0787 | - | - |
| Relative Molecular Mass (Hydrate) | 241.2 | - | - |
| Relative Molecular Mass (Anhydrous Salt) | 205.2 | - | - |
| Appearance Of Solution | Clear and colourless | - | Eur. Ph. |
| Water | max. 15.0 | % | Eur. Ph. |
| Content (Dry Basis) | 99.0 – 101.0 | % | PRO.49 . |
| pH (Solution 10% In Water) | 6.0 – 7.5 | - | - |
| Acidity or Alkalinity | Pass the test. | - | Eur. Ph. |
| Benzoates And Salicylates | Not detectable | - | Regulation 231/2012/CE |
| Elemental Impurities: Conform | ICH Guideline Q3D | - | ICP-MS. |
| Lead | max. 0.5 | ppm | ICP-MS. |
| Selenium | max. 15 | ppm | ICP-MS. |
| Arsenic | max. 1.5 | ppm | ICP-MS. |
| Readily Carbonizable Substances | Pass the test. | - | Ph. Eur. |
| p-TSA (Toluenesulphonamide) | max. 10 | - | Ph. Eur. |
| o-TSA (Toluenesulphonamide) | max. 10 | ppm | Ph. Eur. |
| 1,2-benzisothiazolin-3-one (BIT) | max. 5 | ppm | PRO.87 |
| Methyl Anthranilate | max. 1 | ppm | PRO.87 |
| p-Sulphonamidobenzoic acid | max. 10 | - | PRO.103 |
| Organic Volatiles Impurities | Pass the test | - | U.S.P. |
Regulatory & Compliance
- Certifications & Compliance
- Grade
- Certifications
Certified ISO 9001, ISO 14000 and ISO 22000 + PAS 220,Kosher and Halal certified.
Our sodium saccharin complies with the following purity regulations:
- Pharmaceutical: EP, USP and JP (latest versions)
- Food: European directive 2008/60/EU and FCC
Packaging & Availability
- Availability
Apart from standard sodium saccharin, we can also offer specialties to meet specific requirements of our customers, with for example specific mesh-sizes, water-content, higher purity, etc..
- Packaging
- Boxes 25 Kg
- Drums 50 Kg
Storage & Handling
- Shelf Life
- 5 Years

