Knowde Enhanced TDS
Identification & Functionality
- Active Component
- Chemical Family
- INCI Name
- Ingredient Name
- Ingredient Origin
- Cosmetic Ingredients Functions
- CAS No.
- 13956-29-1
Features & Benefits
- Benefit Claims
- Benefit Claims (Health)
- Labeling Claims
- Food Ingredients Features
- Additives and Preservatives
PureForm CBD™ does not contain additives and is made without preservatives.
- Product Benefits
- Anti inflammation
- Arthritis support
- Anti anxiety
- Sleep support
- Anti eizure
- Acne support
- Addiction treatment
- Food Ingredient Features
- THC-free
- Citrus Derived
- Pesticide-free
- FSMA 117
- cGMP Manufactured
- GRAS Certified
- 99.5%+ Pure
- Non-GMO
- Clean Label Project Certified
- Not made from Cannabis or Hemp
- Patented
- 100% Nature-Identical
- Available in commercial scale quantities
- US & UK manufacturing sites
- PureForm's Environmental Commitment Through Sustainability
Our Cyclic Terpene Assembly (CTA) technology delivers a high-performance alternative to agricultural and biosynthetic-based production of molecules in a cost-efficient and sustainable format.
Key Environmental Comparison
PureForm’s CTA process vs industrial farming and fermentation-based cannabinoid production.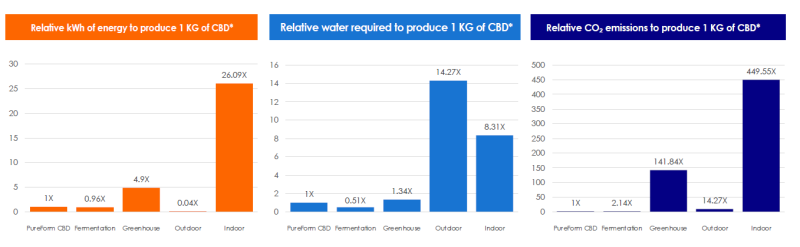
Applications & Uses
- Applications
- Application Format
- Food & Nutrition Applications
- Hair Care Applications
- Treatment Product Applications
- Product Uses
PureForm CBD™ can be added to a wide variety of foods, beverages, nutritional supplements, cosmetics, topicals, and pet food and treats. It is available in a crystalline powder format, or solubilized in water or oil formats.
- Suggested Delivery System
- Sublingual tabs
- Effervescent tablets
- Gel capsules
- Vitamin capsules
- Protein shakes
- Food ingredients
- Liquid tinctures
- Serving jars/pouche
- Topicals
- Tablets
- Sprays
- Shampoo
- Body salves
- Beverages
- Paper packets
- Vaping oils
Properties
- Appearance
- White to tan or beige solid
- Microbiological Values
- Heavy Metals
- Note
- *No Class 1 or Class 2 solvents were utilized during the manufacture, processing, or handling of CBD.
- Generally Recognized as Safe (GRAS) is an FDA designation that a chemical or substance added to food is considered safe by experts.
- PureForm CBD™ is derived from non-cannabis botanical sources. No cannabis-derived material was used in the production of this cannabidiol.
- Note
*The % Daily Value (DV) tells you how much a nutrient in a serving of food contributes to a daily diet. 2,000 calories a day is used for general nutrition advice.
| Value | Units | Test Method / Conditions | |
| E. coli | Absent | — | — |
| Total Aerobic Microbial Count | max. 1000 | CFU/g | — |
| Total Yeast & Molds | max. 100 | CFU/g | — |
| Value | Units | Test Method / Conditions | |
| Arsenic | max. 5 | ppm | — |
| Cadmium | max. 5 | ppm | — |
| Lead | max. 5 | ppm | — |
| Mercury | max. 5 | ppm | — |
| Zinc | max. 1300 | ppm | — |
Regulatory & Compliance
- Certifications & Compliance
- Regulatory Status
FDA-Audited MFG facility, Generally Recognized As Safe (GRAS), Produced under the cGMP guidelines outlined in Section 117 of the Food Safety & Modernization Act (FSMA).
- Chemicals Information
The following chemicals of the European COLIPA guidelines are expressly declared:
Ingredients Directly
Added (%)Essential Oil
Contribution (%)As Impurity
(%)Amyl Cinnamic Alcohol 0 0 0 Amyl Cinnamic Aldehyde 0 0 0 Hexyl Cinnamic Aldehyde 0 0 0 Hydroxycitronellal 0 0 0 Lilial 0 0 0 Lyral 0 0 0 Methyl Heptyne Carbonate 0 0 0 Methyl Octyne Carbonate 0 0 0 Methyl Ionone Iso/Alpha 0 0 0 Phenyl Acetaldehyde 0 0 0 Anisyl Alcohol 0 0 0 Benzyl Alcohol 0 0 0 Benzyl Benzoate 0 0 0 Benzyl Cinnamate 0 0 0 Benzyl Salicylate 0 0 0 Cinnamic Alcohol 0 0 0 Cinnamic Aldehyde 0 0 0 Citral (Neral + Geranial) 0 0 0 Citronellol 0 0 0 Coumarin 0 0 0 Estragole (Methyl Chavicol) 0 0 0 Eugenol 0 0 0 Farnesol 0 0 0 Geraniol 0 0 0 Isoeugenol 0 0 0 Limonene (D, L, and DL) 0 0 max. 0.1% Linalool 0 0 0 Methyl Eugenol 0 0 0 Oakmoss 0 0 0 Treemoss 0 0 0 - Gluten Statement
PureForm CBD™ does not originate from gluten containing cereals and does not identify as containing gluten.
- GMO Statement
PureForm CBD™ is non-GMO. It is produced from ingredients that
are certified as not genetically-engineered or genetically-modified.- GRAS Statement
PureForm CBD™ is generally recognized as safe (GRAS) as an ingredient in non-alcoholic beverages, nutrition beverages and bars, chocolate, and dietary supplements, per an estimated daily intake of 50.5 mg/person for food and supplement ingredient use
- Organic Statement
CBD™ is not certified as organic
- Food-Grade Compliance
PureForm CBD™ is manufactured under current Good Manufacturing Practices (cGMP) guidelines outlined in section 117 of the Food Safety & Modernization Act (FSMA), delivering purity that is sufficiently high to ensure safety under customary conditions of intentional use in foods and foods processing.
- Heavy Metal
PureForm CBD™ is manufactured via Cyclic Terpene Assembly from raw materials that are principally derived from natural sources. No class 1, 2 and 3 metals are used in the manufacturing process of the product. Heavy metals content in the product is monitored as part of release testing, per limits specified in the U.S. Pharmacopeia chapter <232> on Elemental Impurities and ICH Guideline Q3D
- Residual Solvents
This statement is to certify that no Class 1 or 2 residual solvents as listed in the U.S. Pharmacopeia General Chapter <467> and the ICH Harmonized Guideline for Residual Solvents Q3C are used in the manufacture of PureForm CBD™.
- Melamine-Free Statement
- PureForm CBD™ is Melamine-free in accordance with U.S. FDA Guidance for Industry for Pharmaceutical Components at Risk fo melamine contamination.
- The manufacturing process for PureForm CBD™ does not use any at-risk components of melamine contamination as identified in the referenced guidance
- BSE & TSE Statement
- PureForm CBD™ is not of animal origin and does not contain any raw materials produced from, or substances derived from animal origin. The manufacturing process does not use any ingredient of animal origin.
- PureForm CBD™ is stored and transported in a manner that prevents contact with products of animal origin.
- PureForm CBD™ is certified to be free from Transmissible Spongiform Encephalopathy (TSE) and Bovine Spongiform Encephalopathy (BSE).
- Note
PureForm does not make any claims regarding these effects. These statements have not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat, cure, or prevent any disease. These statements are based solely on review of published scientific literature.
Technical Details & Test Data
- Pesticides Test
Analysis for over 60 pesticides was undertaken using the following methods: AOAC official method 2007.01, Pesticide residues in Foods by Acetonitrile Extraction and partitioning with Magnesium Sulfate, AOAC INTERNATIONAL
CEN Standard Method EN 15662: Food of plant origin - Determination of pesticide residues using GC-MS and/or LC-MS/MS following acetonitrile extraction/partitioning and clean-up by dispersive SPE-QuEChERS method.
Analysis Results Abamectin <0.05 mg/kg Aldicarb <0.05 mg/kg Aldicarb sulfone (Aldoxycarb) <0.05 mg/kg Aldicarb sulfoxide <0.05 mg/kg Azoxystrobin <0.05 mg/kg Bifenazate <0.05 mg/kg Bifenthrin <0.05 mg/kg Carbaryl <0.05 mg/kg Carbofuran <0.05 mg/kg Carbofuran-3-hydroxy- <0.05 mg/kg Chlorantraniliprole <0.05 mg/kg Chlordane, cis- <0.05 mg/kg Chlordane, trans- <0.05 mg/kg Chlorfenapyr <0.05 mg/kg Chlorpyrifos <0.05 mg/kg Coumaphos <0.05 mg/kg Cyfluthrin <0.05 mg/kg Cyproconazole (2 diastereoisomers) <0.05 mg/kg Cyprodinil <0.05 mg/kg Dichlorvos <0.05 mg/kg Diclobutrazol <0.05 mg/kg Dipropetryn <0.05 mg/kg Disulfoton <0.05 mg/kg Endosulfan I (alpha-isomer) <0.05 mg/kg Endosulfan II (beta-isomer) <0.05 mg/kg Endosulfan sulfate <0.05 mg/kg Epoxiconazole <0.05 mg/kg Ethiofencarb <0.05 mg/kg Etofenprox <0.05 mg/kg Etoxazole <0.05 mg/kg Fenoxycarb <0.05 mg/kg Fenpropathrin <0.05 mg/kg Fenvalerate/Esfenvalerate (sum of isomers) Fipronil <0.05 mg/kg Fipronil desulfinyl <0.05 mg/kg Fipronil sulfone <0.05 mg/kg Imazalil <0.05 mg/kg Imidacloprid <0.05 mg/kg Malathion <0.05 mg/kg Methiocarb <0.05 mg/kg Methiocarb sulfone <0.05 mg/kg Methiocarb sulfoxide <0.05 mg/kg Methomyl <0.05 mg/kg Mevinphos (E- and Z-isomers) Myclobutanil <0.05 mg/kg Naled (Dibrom) <0.05 mg/kg Paclobutrazol <0.05 mg/kg Permethrin (sum of isomers) <0.05 mg/kg Propoxur <0.05 mg/kg Spinetoram (spinosyns J and L) Spinosad
(spinosyns A and D) Spirodiclofen<0.05 mg/kg Spiromesifen <0.05 mg/kg Spiromesifen enol <0.05 mg/kg Spirotetramat <0.05 mg/kg Spiroxamine (2 diastereoisomers) Tebuco-nazole <0.05 mg/kg Thiabendazole <0.05 mg/kg Fipronil desulfinyl <0.05 mg/kg Thiabendazole-5-hydroxy- <0.05 mg/kg Thiacloprid <0.05 mg/kg Trifloxystrobin <0.05 mg/kg Metolachlor <0.05 mg/kg Pyrethrum (total) <0.05 mg/kg
Safety & Health
- Safety Information
PureForm CBD™ is manufactured in FDA-audited facilities and is always pharmaceutical grade. Every step of the way, we perform rigorous testing to ensure PureForm CBD™ is the safest, purest product on the market. No THC, no neurotoxins, no pesticides, no heavy metals, no mycotoxins. No harmful contaminants, period.
Packaging & Availability
- Packaging Information
- 5g
- 20g
- 100g
- 1kg
Storage & Handling
- Storage Information
- Store long term at refrigerated temperature between 2 - 8°C.
- Avoid contact with moisture and UV light.
- Store in theoriginal, airtight container.
