Knowde Enhanced TDS
Identification & Functionality
- INCI Name
- Cosmetic Ingredients Functions
- Molecular formula
- C₂₆H₃₈Cl₂N₁₀O₄
- EC No.
- 200-302-4
- CAS No.
- 56-95-1
- Technologies
- Product Families
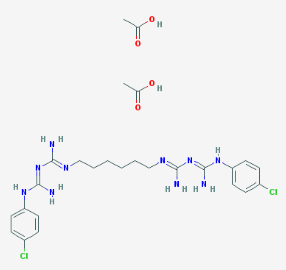
- Structure
Features & Benefits
Applications & Uses
- Markets
- Uses
- Chlorhexidine diacetate is used as an ingredient of bacteriostatic and bacteriocidal products to provide the activity of membrane disruption.
- Chlorhexidine acetate is a high quality antiseptic used in broad filed of indications.
- Disinfectant -General Disinfection of equipment, surfaces and textiles
- Veterinary uses - Chlorhexidine Acetate is also used for many veterinary applications in certain geographical areas instead of Chlorhexidine Di Gluconate
- Others - Chlorhexidine Acetate is used to manufacture antacid tablets.
Properties
- Physical Form
- Appearance
- White or almost white crystalline powder
- Typical Properties
- Solubility
Sparingly soluble in water, soluble in ethanol 96%, slightly soluble in glycerol.
| Value | Units | Test Method / Conditions | |
| Loss On Drying | max. 1.0 | % | — |
| Assay (Dried Basis) | 98.0 - 102.0 | % | — |
| Molecular Weight | 625.556 | g/mol | — |
Regulatory & Compliance
- Certifications & Compliance
- Specification At Release
EP/USP as per requirement.
- Regulatory Status
Chlorhexidine acetate matches the high quality regulatory standards of eu authorities, a dmf is available.
API (Ph. Eur.)
- GMP (Good Manufacturing Practice)
- DMF (Drug Master File) issued 2019
Cosmetic
- Chlorhexidine diacetate is registered in the CosIng (Cosmetic Ingredient Database).
- This means that it can be used in cosmetics as a preservative with a maximum concentration of 0.3%.
Packaging & Availability
- Packaging Type
- Packaging
Drums of 25kg, other packaging on request.
Storage & Handling
- Stability
- 36 months
- Storage Conditions
- Store at controlled temperature (15-30C)
- Keep in the original drums tightly closed
- Keep the drums away from direct sunlight

