Knowde Enhanced TDS
Identification & Functionality
- Chemical Family
- Chemical Name
- INCI Name
- Cosmetic Ingredients Functions
- CAS No.
- 36062-04-1
- EC No.
- 609-201-3
- Technologies
- Product Families
- Product Details
- Product code : 2032
- Category : Intended for Cosmeceutical application
- Preparation type : Synthetic
- Molecular Formula : C21H24O6
- Chemical Name : Tetrahydrodiferuloylmethane
- Starting Material : Vanillin and Acetylacetone
- Synonyms : 1,7-Bis(4-hydroxy-3-methoxyphenyl)-3,5- heptanedione
- Excipient used : None
Features & Benefits
- Labeling Claims
- Product Highlights
TetraPure®, an excellent ingre- dient for cosmetic formulations with proven efficacy against a wide range of fungi. TetraPure® has been standardized for a minimum of 99% of Tetrahydrocurcumin (1, 7-Bis (4- hydroxy-3-methoxyphenyl)-3, 5- heptanedione). It is known to exhibit similar physiological and pharmacological actions as curcumin, in some cases even better. Laboratory studies have shown that TetraPure® can be used for the management of superficial and cutaneous mycoses as well as with multifunctional topical benefits such as antioxidant and skin lightening properties.
Applications & Uses
- Markets
- Applications
- Skin Care Applications
- Applications
- Antioxidant
- Skin Lightening
- Antifungal
- Formulation Guidelines
- Use stainless steel (SS316) vessels for production. Avoid contact with iron or copper
- Solubilize TetraPure® in one of the recommended solvents before adding it to emulsion below 40°C
- Nonionic and anionic emulsifiers are to be used
- Maintain the pH of the formulation slightly acidic, preferably between pH 5.0 - 6.0
- TetraPure® can be gelled using thickeners such as carbomer, acrylates copolymer and lecithin
- Opaque packaging is recommended for the finished product formulation
- Protect the finished product from exposure to direct heat and light
- TetraPure® is suitable for use in creams and translucent to opaque gels/ serums
- TetraPure® is not suitable for use in clear gels/serums/toners
- Use antioxidants and photostabilizers in the formulations
- Suggested Use Level
- Antifungal/Anti-dandruff formulation : 0.15% w/w
- Skin lightening/Antioxidant formulation : 0.1 - 0.5% w/w
Properties
- Appearance
- White to off white crystalline powder
- Soluble in
- Alcohol, Acetone, Glacial acetic acid
- Insoluble in
- Water
- Chemical Properties
- Physical Properties
- Typical Properties
- Other Properties
- Microbiological Values
- Solubility Data
Sage Extract Soluble N-Methyl-2-Pyrrolidone 1:2 at 40°C Ethanol 1:5 at 40°C Eldew SL-205 (Ajinomotto) 1:10 at 50°C Propylene glycol 1:10 at 50°C
| Value | Units | Test Method / Conditions | |
| Tetrahydrocurcumin Content (HPLC - on dry basis) | min. 99.0 | % w/w | SLL/STP-T-006 |
| Value | Units | Test Method / Conditions | |
| Loss on Drying (Dried at 60°C, Under Vacuum) | max. 1.0 | % w/w | USP <731> |
| Loose Bulk Density | 0.30 - 0.60 | g/ml | USP <616> |
| Sieve Test (Passes Through #20 Mesh) | min. 100 | % w/w | USP <786> |
| Sieve Test (Passes Through #40 Mesh) | min. 70 | % w/w | USP <786> |
| Melting Range | 97 - 99 | °C | USP <741> |
| Sieve Test (Passes Through #80 Mesh) | min. 50 | % w/w | USP <786> |
| Residue on Ignition | max. 1.0 | % w/w | USP <281> |
| Tapped Bulk Density | 0.40 - 0.90 | g/ml | USP <616> |
| Value | Units | Test Method / Conditions | |
| Molecular Weight | 372.41 | — | — |
| Value | Units | Test Method / Conditions | |
| Arsenic Content | max. 1 | ppm (μg/g) | ICP-OES, SLL/STP-H-006 |
| Cadmium Content | max. 1 | ppm (μg/g) | ICP-OES, SLL/STP-H-006 |
| Lead Content | max. 3 | ppm (μg/g) | ICP-OES, SLL/STP-H-006 |
| Mercury Content | max. 0.1 | ppm (μg/g) | ICP-OES, SLL/STP-H-006 |
| Value | Units | Test Method / Conditions | |
| Coliforms Count | max. 10 | cfu/g | BAM 2001, 8th Edition, Chapter 4 |
| Escherichia Coli | Negative | per 10g | USP <62> |
| Pseudomonas Aeruginosa | Negative | per 10g | USP <62> |
| Salmonella | Negative | per 10g | USP <62> |
| Staphylococcus Aureus | Negative | per 10g | USP <62> |
| Total Aerobic Microbial Count | max. 5000 | cfu/g | USP <61> |
| Total Yeasts and Molds Count | max. 100 | cfu/g | USP <61> |
Technical Details & Test Data
- Treatment of Superficial and Cutaneous Mycoses
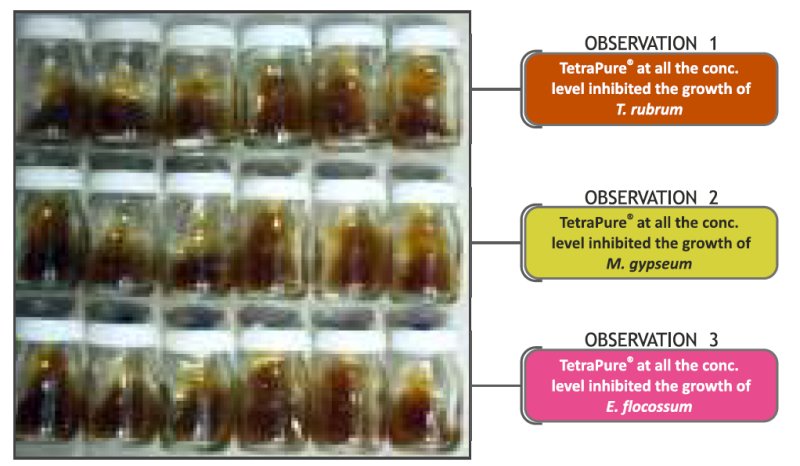
Antifungal Efficacy
TetraPure® at concentrations of 0.15%, 0.31%,0.625%, 1.25%, 2.5% & 5% completely inhibits the growth of dermatophytes Trichophyton rubrum, Microsporum gypseum and Epidermophyton flocossum both at low and high concentrations.
Anti Dermatophytic activity of TetraPure®
Activity Against Candida Species
At 0.15% TetraPure® reduces the colony counts of Candida albicans NCIM3471 to less than 100 cfu/ml with an overall percentage reduction of 99.99% over atest interval time of 28 days.Test Organism Test interval Concentration of organism (days) % Reduction (cfu/ml) Candida albicans NCIM 3471 (yeast) 0 14.6 x 10⁵ - 7 72 x 10⁵ 50.6 14 20 x 10⁸ 99.8 21 75 x 10² 99.94 28 <130 99.99
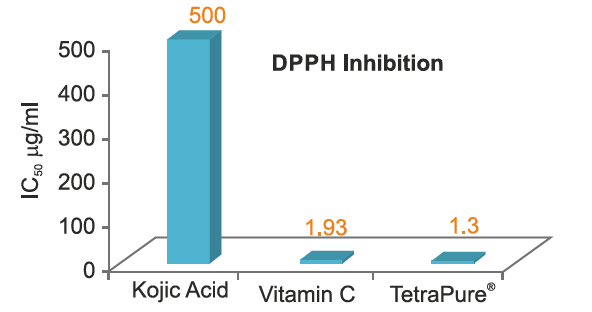
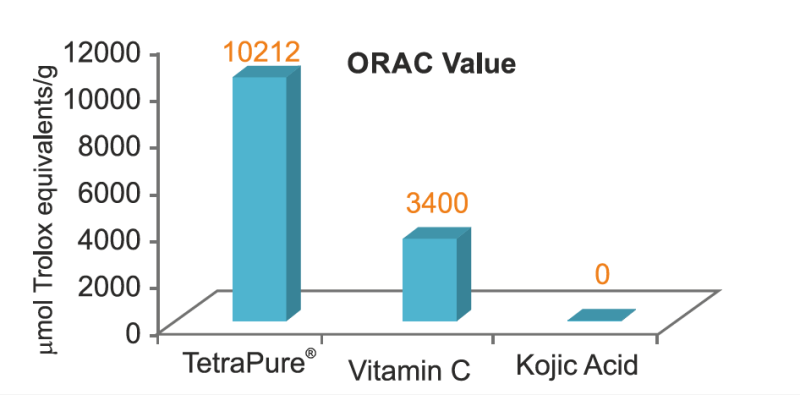
Antioxidant Potential
In vitro data reveal that TetraPure® efficiently scavenges free radicals and protects the skin cells. The free radical scavenging activity of TetraPure® was found to be superior to that of Vitamin C and Kojic acid.
Comparison of Antioxidant Potential
Lower the IC,, value, greater the antioxidant activity
Greater the ORAC value, better the antioxidant activity
Anti-dandruff Activity
TetraPure® at concentrations of 0.15%, 0.31%, 0.625%, 1.25%, 2.5% and 5.0%, inhibited the activity of Malassezia furfur, the dandruff causing fungus.
Skin Lightening Potential
In vitro studies indicate that TetraPure® efficiently inhibits tyrosinase, the rate limiting enzyme inthe synthesis of melanin. Its efficacy is superior to that of commonly used natural skin lightening agents such as Kojic acid and of related compounds.
Lower the IC₅₀ value, greater the skin lightening activity.Product Tyrosinase Inhibition
(IC₅₀ µg/ml)Melanin Inhibition
(IC₅₀ µg/ml)Tetrapure® 18 3.2 Vitamin C 9.33 25 Kojic Acid 7 100 Arbutin 193.6 100
Storage & Handling
- Shelf Life
- 5 Years
- Storage Condition
- Store at room temperature.

