Knowde Enhanced TDS
Identification & Functionality
- Chemical Name
- INCI Name
- Cleaning Ingredients Functions
- Cosmetic Ingredients Functions
- CAS No.
- 68650-39-5
- EC No.
- 272-043-5
- Technologies
- Product Families
- Chemical Structure
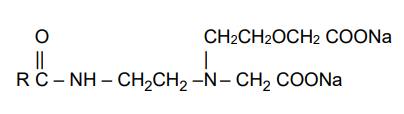
R = coco
- Preservative
DMDM Hydantoin
Features & Benefits
- Benefit Claims
- Labeling Claims
- HII Features
- Functional Properties
- Secondary surfactant
- Foam booster
- Viscosity builder
- Compatible with anionics, nonionics and cationics.
- Biodegradability
Product is readily biodegradable.
Applications & Uses
- Markets
- Applications
- Bath & Shower Applications
- Home Care Applications
- Skin Care Applications
- End Product Uses
- Bubble Baths
- Body Washes
- Shampoos
- Baby Products
- Pet Shampoos
- Hand Soaps
- Facial Washes
- Shower Gels
- Ethnic Products
Properties
- Physical Form
- Appearance
- Clear liquid (at 25°C)
- Typical Properties
| Value | Units | Test Method / Conditions | |
| Solids Content | 50 | % | — |
| Color | 3 | — | Gardener Scale |
| Density | 1.09 | g/ml | — |
| pH (10% in aqueous solution) | 8.5 | — | — |
| Freeze Point | -15 | °C | — |
| Regulated Volatile Organic Chemicals | 0 | % | U.S. EPA |
| Actives Content | 38 | % | — |
| Sodium Chloride Content | 11.5 | % | — |
| Specific Gravity (at 25°C) | 1.09 | — | — |
Regulatory & Compliance
- Certifications & Compliance
- Chemical Inventories
- Clearances
The international inventories (country clearances) of AMPHOSOL 2C can be found in Section 15 of the Safety Data Sheet (SDS). It is the responsibility of the formulator to review the chemical control regulations for each country where the end product is intended to be sold or used.
Safety & Health
- Health Effects
Non-irritating to the eyes at concentration of 5% active (Draize OECD 405), and to the skin at a concentration of 10% active (Draize OECD 404).
Packaging & Availability
- Standard Packaging
AMPHOSOL 2C is available in bulk, tote and drum quantities.
Storage & Handling
- Storage & Handling
- Normal safety precautions (i.e. gloves and safety goggles) should be employed when handling AMPHOSOL 2C in its concentrated form. Contact with the eyes and prolonged contact with the skin should be avoided. Wash thoroughly after handling material.
- It is recommended that AMPHOSOL 2C be stored in sealed containers and kept at temperatures between 50°F (10°C) and 120°F (49°C). Avoid overheating or freezing. If material is frozen, mild heat and agitation are recommended to ensure the material is homogeneous before use.
- Workplace Exposure
Occupational exposure can occur primarily through skin contact or via inhalation of vapors and mists. Engineering controls, personal protective equipment, and other workplace safety practices should be used to control these exposures. See SDS for more information.

