Knowde Enhanced TDS
Identification & Functionality
- Chemical Family
- Ingredient Origin
- Food Ingredients Functions
- Ingredients
- Salt, Wheat Flour, Asparaginase
- Technologies
Features & Benefits
- Labeling Claims
- Food Ingredients Features
Applications & Uses
- Markets
- Food & Nutrition Applications
- Application Examples
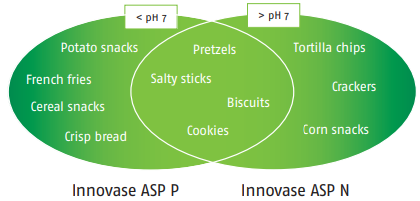
Innovase ASP P for potato snacks
The potato is a rich source of asparagine and therefore very susceptible to the formation of acrylamide.
The Regulation (EU) 2017/2158 benchmark for potato snacks is 750 ppb. Dosage: We recommend a dosage of 500-1000 ppm based on dry matter.
Application: Innovase ASP P is added together with the water or other ingredients prior to mixing. The most cost-efficient enzyme dosage can be realized within the optimum temperature range of 40-60°C and a reaction time of 30 min.
Innovase ASP P for biscuits
Semi-hard biscuits are baked at high temperatures to a very low final product humidity. Thus, acrylamide formation is very likely.
The Regulation (EU) 2017/2158 benchmark for semi-hard biscuits is 150 ppb. Dosage: We recommend a dosage of 150-300 ppm based on flour weight.
Application: Innovase ASP P is added together with the water or flour prior to mixing. No additional adjustments required.
Innovase ASP N for crackers
The use of ammonium bicarbonate for pH adjustment and as a leavening agent contributes to acrylamide formation.
The Regulation (EU) 2017/2158 benchmark for soda crackers is 400 ppb. Dosage: We recommend a dosage of 50-150 ppm based on flour weight.
Application: Innovase ASP N is added together with the flour and the sponge dough to the mixer resting stage
Properties
- Appearance
- Powder
- Odor
- Neutral to fermented
- Chemical Properties
- Typical Properties
- Microbiological Values
- Nutritional Information
- Nutritional Data
Description Value Unit/100g Energy 1.387 KJ Energy 327 kcal Fat 1 g of which: saturated fatty acids (FA) 0 g Carbohydrates 66 g of which: Sugar 0 g Fiber 3 g Protein (N x 6,25) 12 g Salt (Sodium x 2.5) 7,8 g Sodium 3,12 g - The nutritional information is calculated according to EC regulation 1169/2011.
- The nutritional information is calculated according to EC regulation 1169/2011.
| Value | Units | Test Method / Conditions | |
| Arsenic Content | max. 3 | mg/kg | AAS |
| Cadmium Content | max. 0.5 | mg/kg | AAS |
| Lead Content | max. 5 | mg/kg | AAS |
| Loss on Drying | max. 14 | g/100g | §64 LFGB-2h 130°C |
| Mercury Content | max. 0.5 | mg/kg | AAS |
| Value | Units | Test Method / Conditions | |
| Asparaginase | 1000 - 1300 | u/g | — |
| Value | Units | Test Method / Conditions | |
| Escherichia coli | max. 10 | cfu/g | M. Colif.-Agar 36°C/48h |
| Salmonella | Negative | cfu/25g | DIN EN ISO 6579 |
| Total Plate Count | max. 50000 | cfu/g | DIN EN ISO 4833 |
| Value | Units | Test Method / Conditions | |
| Energy | 1.387 | kJ/100 g | — |
| Fat Content | 1 | g/100g | — |
| Saturated Fatty Acids | 0 | g/100g | — |
| Carbohydrates | 66 | g/100g | — |
| Sugar | 0 | g/100g | — |
| Fibre | 3 | g/100g | — |
| Protein (N x 6.25) | 12 | g/100g | — |
| Salt (Sodium x 2.5) | 7.8 | g/100g | — |
| Sodium Content | 3.12 | g/100g | — |
Regulatory & Compliance
- Regulatory Information
Allergen Information: contains the allergen gluten according to Regulation 1169/2011/EG, Annex II Health Information: The products are suitable for human consumption if used accordingly to recommendations and officially allowed to be sold within Germany and the EU. The product is food grade and conforms with the current european food law.
GMO: This product is not a genetically modified organism (GMO), nor does it contain any GMO or any recombinant DNA.
All the non-enzymatic constituents of the product (e.g. carriers, stabilizing agents, free-flowing agents etc.) originate from non-genetically modified sources. Due to the ubiquitous presence of GMO organisms, absence of minor traces of GMO material cannot be guaranteed, but in any case, the content of unintentionally present GMO material will be below 0.9 %, in accordance to EU guidelines 1830/2003 and 1829/2003. The genetic sequence of the enzyme protein was multiplied and introduced into the production organism using modern biotechnological methods. The product is used as processing aid in food manufacturing processes, and therefore labeling is not required in the EU. National regulations must be observed.
Yes No Vegetarian X Vegan X BSE/TSE Information: The mentioned product does not represent a BSE (Bovine Spongiform Encephalopathy) and/or TSE (Transmissible Spongiform Encephalopathy) risk.
Irradiation: Neither the product nor its ingredients have been treated with ionizing radiation according to Directive 1999/2/EC and 1999/3/EC.
Nanoparticles: The product does not contain ingredients in the form of engineered nanomaterials as defined in Regulation (EU) 2015/2283.
Contamination/Residues: The product complies with: Regulation (EC) No 396/2005 and its amendments regarding maximum levels for pesticide residues in foodstuffs. Regulation (EC) No 1881/2006 and its amendments regarding especially maximum levels for heavy metals, mycotoxins, dioxins and PCB in foodstuffs.
Conformity of the Packaging: The packaging material complies with the provisions of Regulation (EC) No 1935/2004 and Regulation (EU) No 10/2011.
Technical Details & Test Data
- Application Notes
- Efficient acrylamide reduction
- Only minor process or recipe adjustments
- Unchanged taste and texture of the end products
- Applicable to a wide range of products
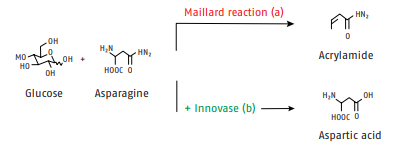
Enzyme system for acrylamide reduction Innovase ASP The formation of acrylamide is a result of the Maillard reaction between reducing sugars and asparagine, a naturally occurring amino acid. Unfortunately, acrylamide is suspected of being a potential carcinogen and is thus considered a process contaminant. This has led to almost worldwide regulations to reduce acrylamide in food products. Proven mitigation strategies usually include drastic changes in process conditions and raw material selection, both of which risk undesirable changes in product properties. Innovase ASP, on the other hand, removes asparagine from the Maillard reaction with little, if any, process adjustments (see Fig. 1)
Pathways of the Maillard and asparaginase reaction
Fig. 1: Pathway of the Maillard reaction of glucose and asparagine to acrylamide (a) and the alternative reaction pathway if asparaginase is used to prevent acrylamide formation (b).
Two versions for a perfect product fit Innovase ASP catalyzes the decomposition of the amino acid asparagine to aspartic acid, thus preventing the formation of acrylamide. In order to find the best possible solution, we offer two variants of asparaginase. Both can be used up to 60 °C, but differ in their pH optima to provide the most suitable solution depending on the existing process pH (see Figure 2).
Fig. 2: Innovase ASP choice depending on the pH of the food product during processing
Packaging & Availability
- Packaging
PE bag in cardboard box, 20 kg net weight
Storage & Handling
- Storage & Shelf Life
Min. 12 months if stored cool and dry in closed original packing







