Knowde Enhanced TDS
Identification & Functionality
- INCI Name
- Vitamin Type
- Cosmetic Ingredients Functions
- Encapsulated Material
- Ascorbyl Tetraisopalmitate
- Technologies
Features & Benefits
- Benefit Claims
- Labeling Claims
Applications & Uses
- Markets
- Application Format
- Skin Care Applications
- Use Level
- 1 - 4%
- Application
Skin Care, Color cosmetics
- Processing
Add at the last stage of formulation preparation, using a paddle mixture, below 40°C degrees
Properties
- Appearance
- White Powder
- Typical Properties
| Value | Units | Test Method / Conditions | |
| Loading Capacity | 25 | % | — |
| NOI | 0.65 | — | — |
Technical Details & Test Data
- Micrograph
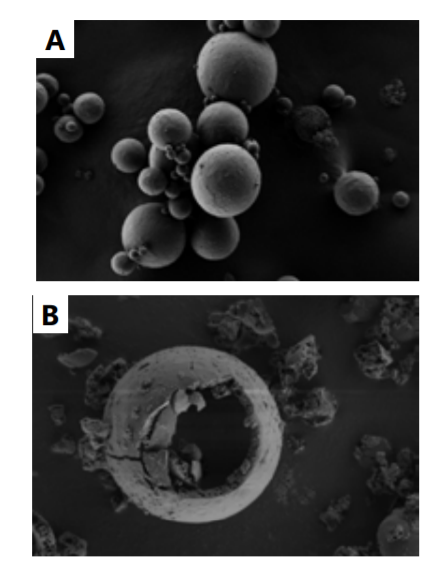
Fig. 1.A. SEM micrograph of Ascorbyl Tetraisopalmitate microcapsules, CelluCapTM C. B. SEM observation demonstrating the Release on Demand™ (RND™) technology by applying mechanical pressure.
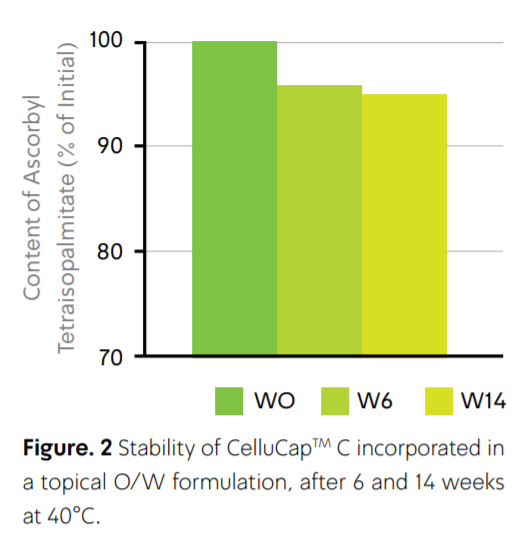
- Formulation Advantages
Tagra’s unique delivery system ensures optimal isolation of the Ascorbyl Tetraisopalmitate resulting in increase of its stability (Fig. 2), preventing incompatibilities and formulation discoloration. CelluCapTM C appears as a non-agglomerate powder which is easily incorporated into the already made base formulation during the last stage of preparation. The product is compatible with all types of cosmetic formulations, no stabilizers or special equipment required, without any pH limitations. CelluCap™ range complies with all regulatory requirements worldwide.
* A lypophilic derivative of Vitamin C, known also as Tetrahexyldecyl ascorbate, which exhibits excellent percutaneous abilities converting into free Vitamin C within the cells
- Clinical Testing
INCREASE OF SKIN BRIGHTENING
Twelve Asian females (aged 35-60 years old) treated with formulation containing 3% w/w CelluCapTM C demonstrated increase in skin brightening as indicated by measuring melanin levels and ITA° angle. Cream was applied twice daily on face and on both outer forearms. Non-treated areas served as a baseline (t0w).
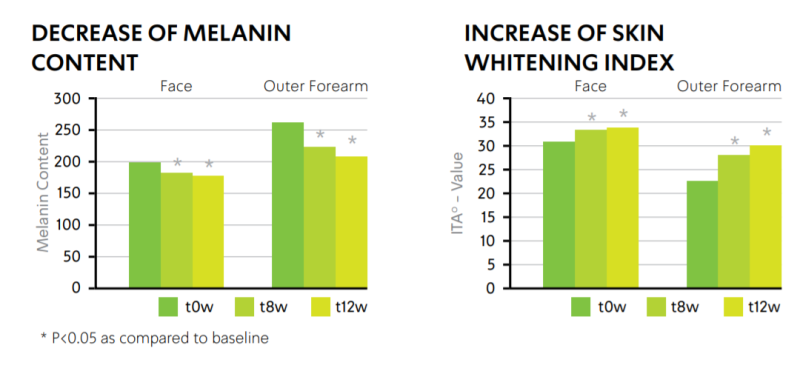
* P<0.05 as compared to baseline
CELLUCAPTM C: SIGNIFICANT WRINKLE REDUCTION
The anti-aging effect of 2% w/w CelluCapTM C resulted in an average 23.22% reduction in skin roughness, as tested on five female volunteers treated with O/W formulation.
CLINICAL EVALUATION: 14 AND 28 DAYS
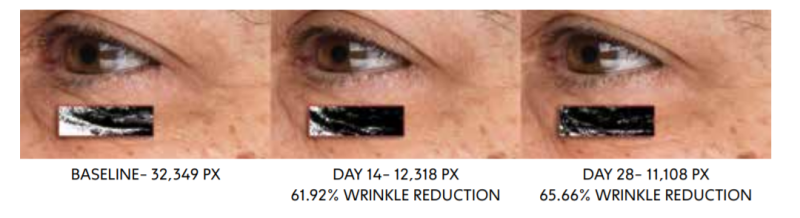 CELLUCAPTM C SMOOTHS SKIN AND IMPROVES MOISTURIZATION
CELLUCAPTM C SMOOTHS SKIN AND IMPROVES MOISTURIZATION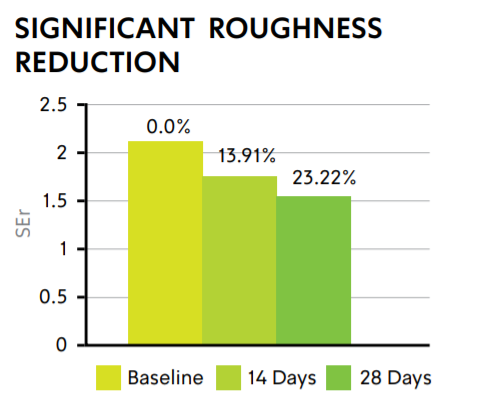
Surface evaluation of living skin via Visioscan demonstrates a decrease in SEr parameter associated with reduction in depth fine lines and wrinkles
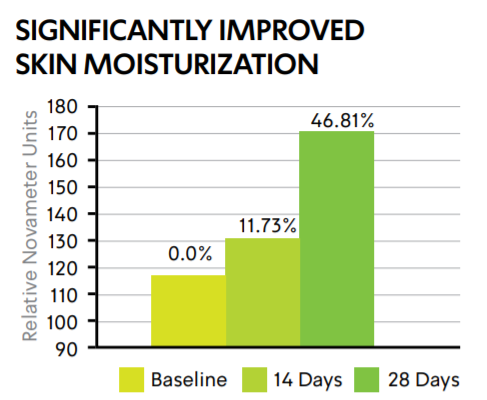
A relative measure of the retained water content of the skin as function of skins dielectric value.

