Knowde Enhanced TDS
Identification & Functionality
- Chemical Name
- CAS No.
- 7664-41-7
- EC No.
- 231-635-3
- Technologies
Features & Benefits
- Product Highlights
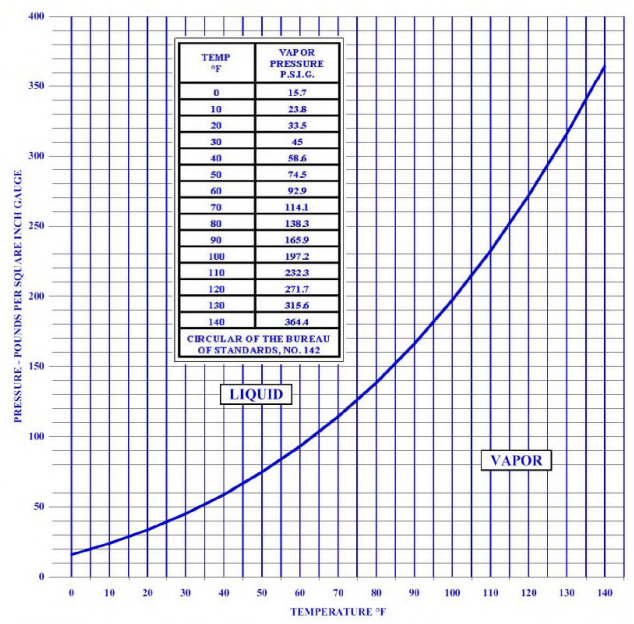
At atmospheric temperature and pressures, anhydrous ammonia is a pungent colorless gas. Anhydrous ammonia boils at -28°F and freezes to a white crystalline mass at -108°F. When heated above its critical temperature of 270.3°F, ammonia exists only as a vapor regardless of the pressure. Between the melting and critical points, liquid ammonia exerts a vapor pressure, which increases with rising temperature. When liquid ammonia is in a closed container, it is in equilibrium with ammonia vapor and the pressure within the container bears a definite relationship to the temperature.
Liquid anhydrous ammonia is lighter than water, having a density of 42.57 pounds per cubic foot at -28°F, while as a vapor, ammonia is lighter than air, its relative density is 0.597 compared to air at atmospheric pressure and a temperature of 32°F. Under the latter conditions, one pound of ammonia vapor occupies a volume of 20.78 cubic feet. At 70°F and at atmospheric pressure, one pound of ammonia vapor occupies a volume of 22.5 cubic feet and yields 45 cubic feet of dissociated gas at a ratio of 25% nitrogen and 75% hydrogen.
Applications & Uses
- Markets
Properties
- Molecular Formula
- NH₃
- Physical Properties
| Value | Units | Test Method / Conditions | |
| Molecular Weight | 17.032 | ||
| Boiling Point (1 Atmosphere) | -28 | °F | |
| Freezing Point (1 Atmosphere) | -108 | °F | |
| Critical Temperature | 270.34 | °F | |
| Critical Pressure | 1647.2 | PSIA | |
| Vapor Density (1 Atmosphere, -28°F) | 0.056697 | lb/Cubic ft | |
| Heat of Combustion | 542 | btu/lb |
Technical Details & Test Data
- Liquid Density
Temp
(°F)Lbs./Cu.Ft. Lbs./U.S. Gal. Spec.Gr.Liq.
Comp.Water
(4°F)Latent Heat
BTU per Lb.-28° 0 42.57 5.69 0.682 589.3 -20° 3.6 42.22 5.64 0.675 583.6 -10° 9 41.78 5.59 0.669 576.4 0° 15.7 41.34 5.53 0.663 568.9 10° 23.8 40.89 5.47 0.656 561.1 20° 33.5 40.43 5.41 0.648 553.1 30° 45 39.96 5.34 0.641 544.8 40° 58.6 39.49 5.28 0.633 536.2 50° 74.5 39 5.21 0.625 527.3 60° 92.9 38.5 5.14 0.617 518.1 65° 103.1 38.25 5.11 0.613 513.4 70° 114.1 38 5.08 0.609 508.6 75° 125.8 37.74 5.04 0.605 503.7 80° 138.3 37.48 5.01 0.6 498.7 85° 151.7 37.21 4.97 0.596 493.6 90° 165.9 36.95 4.94 0.592 488.5 95° 181.1 36.67 4.9 0.588 483.2 100° 197.2 36.4 4.87 0.583 477.8 105° 214.2 36.12 4.83 0.579 472.3 110° 232.3 35.84 4.79 0.573 466.7 115° 251.5 35.55 4.75 0.57 460.9 120° 271.7 35.26 4.71 0.565 455 125° 293.1 34.96 4.67 0.56 448.9 130° 315.6 34.66 4.63 0.555 443 135° 339.4 34.35 4.59 0.55 436 140° 364.4 34.04 4.55 0.545 430 - Vapor Pressure Temperature Relationship