Knowde Enhanced TDS
Identification & Functionality
- Pharma & Nutraceuticals Functions
- Technologies
Features & Benefits
- Product Highlights
DEP® cabazitaxel is the second product from Starpharma’s DEP® platform to enter the clinic, and follows DEP® docetaxel, which delivered positive phase 1 clinical results in 2017 and has progressed to phase 2. The reproducible benefits observed for DEP® docetaxel and DEP® cabazitaxel in preclinical models include decreased bone marrow toxicity and enhanced efficacy, and in both cases DEP® has also allowed for a detergent-free formulation resulting in significant additional benefits for patients.
Starpharma’s DEP® platform was utilized to create DEP® cabazitaxel, a detergent free version of cancer drug Jevtana®. Jevtana® is a leading oncology agent which is used to treat advanced prostate cancer and also under development for other cancers including breast cancer, bladder cancer and head and neck cancer. The current (non-dendrimer) formulation product has US Food and Drug Administration (FDA)-mandated ‘black box’ warnings in relation to neutropenia, which is a major dose limiting side effect, and sever hypersensitivity (e.g. anaphylaxis) resulting from the polysorbate 80 detergent used in its formulation.
DEP® cabazitaxel significantly outperformed Jevtana® in a human breast cancer model with respect to both level and duration of anti-cancer activity and survival, whilst protecting against the development of neutropenia, which is a serious side effect for Jevtana®.
- Advantages
The advantages* of DEP® cabazitaxel include:
- Improved side effect profile
- Detergent-free formulation
- No steroid pre-treatment
- Tumor-targeting
- Improved efficacy
DEP® cabazitaxel has patent filings to 2039 (plus up to an additional ~5 years).
*Multiple preclinical studies have established improved efficacy, survival, and safety with DEP® with many different drugs.
Applications & Uses
- Markets
Technical Details & Test Data
- DEP® cabazitaxel Clinical Status
DEP® cabazitaxel phase 2 program is well advanced. The program is an open-label trial, with the objective of establishing anti-tumor activity (efficacy) & safety.
Encouraging efficacy signals have been observed, including radiological responses, significant target tumor shrinkage and substantial tumor biomarker reductions (e.g. Prostate Specific Antigen - PSA), in cancers including prostate, ovarian, lung, gastroesophageal, head and neck, and other cancers.
- DEP® cabazitaxel Phase 2 - Positive Interim Results in Prostate Cancer Cohort
DEP® cabazitaxel – phase 2 prostate cancer patients
- 25 heavily pre-treated patients (average age 73 years) with Stage (IV) hormone-refractory prostate cancer
- Average of 4 prior anti-cancer treatments and >70 cycles/months
- >95% had received prior taxanes, including docetaxel and cabazitaxel (Jevtana®)
- Patients received DEP® cabazitaxel at a dose of 20mg/m2 cabazitaxel
- No need for prophylactic steroids or antihistamines as polysorbate 80-free aqueous formulation
- No primary G-CSF prophylaxis required
DEP® cabazitaxel – phase 2 interim results in prostate cancer cohort^
- 100% of evaluable patients had one or more efficacy response.
- 64% had prolonged disease control for up to 36 weeks.
- 18% had significant tumor shrinkage, a partial response (Jevtana® – 18.5%)
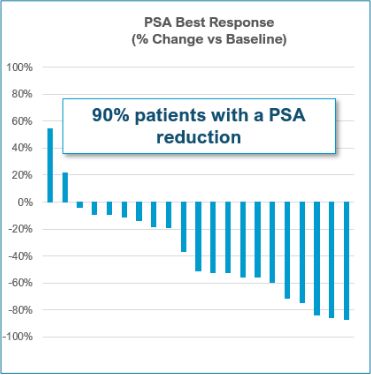
- 90% had a PSA decrease
- 52% had a ≥50% decrease in PSA (Jevtana® – 29.5%)
- 83% had no progression of secondary bone disease
- 56% evaluable for all three of these measures had responses in all three
Efficay Measure DEP® cabazitaxel (20mg/m2) Jevtana® (20mg/m2) PSA Reduction ≥ 50% 52.4% 29.5% Partial Response# 18.2% 18.5% Bone Disease (Improved/Stable) 83.3% Not Reported
Significantly Fewer and Less Severe Adverse Events Reported Than for Jevtana
- Fewer and less severe bone marrow toxicities, particularly neutropenia
- No anaphylaxis observed with DEP® cabazitaxel formulation (aqueous formulation – polysorbate 80-free)
- No severe hypersensitivity or hair loss
- Vast majority of AEs mild to moderate
- Very few patients required G-CSF therapy for myelosuppression
Safety Outcomes DEP® cabazitaxel (20mg/m2) N=25 Jevtana® (20mg/m2) N=598 Neutropenia ≥ grade 3 16.0% 41.8% Febrile Neutropenia ≥ grade 3 0% 2.1% Neutropenia Infection/Sepsis 0% 2.1% - 25 heavily pre-treated patients (average age 73 years) with Stage (IV) hormone-refractory prostate cancer
- DEP® cabazitaxel Case Study in Prostate Cancer
80-Year-Old Man With Stage IV Prostate Cancer
- Progressed following 33 cycles/months of 3 different prior anti-cancer therapies.
- 7 cycles of DEP cabazitaxel to date
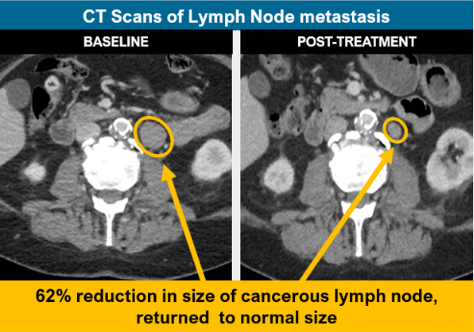
- Achieved 79% reduction in PSA (prostate specific antigen)
- Achieved partial response (significant tumor shrinkage), including a 62% decrease in size of target lymph node)
- No G-CSF therapy required
Notable absence of clinically significant:
- Neutropenia
- Anemia
- Thrombocytopenia
- Other DEP® cabazitaxel Case Studies
DEP cabazitaxel Trial Case Study: 65-Year-Old Man With Late - Stage (Metastatic) Gastro-Oesophageal Cancer
Oesophageal cancer is the seventh most common cause of cancer death among men. The estimated 5-year survival rate for stage IV disease is only 10% to 15%.
- Heavily pre-treated patient with >15 cycles & three different kinds of anti cancer treatment and cancer progressed.
- Response to DEP® cabazitaxel: Patient received 6 cycles of DEP cabazitaxel and achieved a 50% reduction in total tumor size maintained for >27 weeks.
DEP cabazitaxel Trial Case Study: 60-Year-Old Woman With Advanced (Metastatic) Ovarian Cancer
Ovarian cancer has the lowest survival rate of women's cancer and is the eighth most commonly occurring cancer in women.
- Heavily pre-treated; cancer progressed on 3 other anti-cancer therapies including paclitaxel (another taxane); Previously had 14 cycles of treatment and multiple surgeries
- Response to DEP® cabazitaxel: Patient received 6 cycles of DEP cabazitaxel - response seen after 3 cycles of treatment with overall response:
- 40% reduction in total tumor burden for 27 weeks
- 50% reduction in biomarkers
- DEP® cabazitaxel phase 1 results
- 14 patients enrolled and received DEP® cabazitaxel at doses between 2 mg/m2 to 25 mg/m2
- Up to 15 cycles of DEP® cabazitaxel; no steroid, antihistamine or anti-emetic pre-treatment
- Encouraging signs of efficacy were observed in 67% of patients evaluable for treatment response, including:
- Prolonged stable disease in multiple patients and in a variety of cancer types, including prostate, gastro-oesophageal, breast, ovarian, cholangiocarcinoma and pancreatic (& at doses several-fold lower than usually used for cabazitaxel).
- One prostate cancer patient experienced >47 weeks stable disease & a reduction in Prostate Specific Antigen (PSA) of 79%
- One stage IV ovarian cancer patient achieved a reduction in tumor biomarker
(CA-125) of 56% - One stage III cholangiocarcinoma cancer patient achieved a 82% decrease in a tumor biomarker after two cycles
- Prolonged stable disease in multiple patients and in a variety of cancer types, including prostate, gastro-oesophageal, breast, ovarian, cholangiocarcinoma and pancreatic (& at doses several-fold lower than usually used for cabazitaxel).
- Significantly lower levels of side effects usually associated with Jevtana such as bone marrow toxicity (neutropenia, anemia, thrombocytopenia), anorexia and vomiting. No cases of hypersensitivity; no cases of hair-loss; no need for anti-nausea medications
(Evaluable patients are those patients who have received ≥1 dose DEP® cabazitaxel and have had a tumor assessment conducted post treatment)
- DEP® cabazitaxel Preclinical Results
Preclinical studies demonstrated that DEP® cabazitaxel achieved significantly superior anti-cancer effects across a range of important cancer types when compared to Jevtana® (standard cabazitaxel).
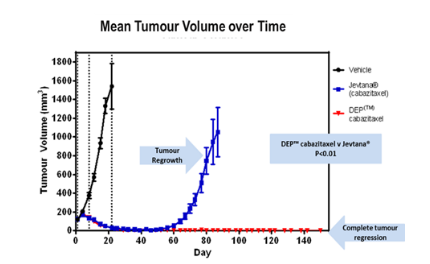
Figure 1: Antitumour activity of DEP® cabazitaxel as compared to Cabazitacel (Jevtana®) in a human breast cancer xenograft model
Starpharma’s DEP® cabazitaxel was compared with Jevtana® in a human breast cancer preclinical model (xenograft). DEP® cabazitaxel significantly outperformed Jevtana® with respect to both level and duration of tumor regression (anticancer activity). Within four weeks of dosing, 100% of mice treated with Starpharma’s DEP® cabazitaxel were tumor free and remained so for the duration of the extended study (150 days). In contrast, the Jevtana® treated group exhibited significant tumor regrowth from day 60 onwards (Figure 1). Tumor growth in both drug treated groups was significantly inhibited compared with the vehicle group (P<0.0001).1
1Statistical analysis of tumor growth inhibition was performed using ANOVA and Dunnett’s post hoc test.

