Knowde Enhanced TDS
Identification & Functionality
- Pharma & Nutraceuticals Functions
- Technologies
- Product Families
- Structure
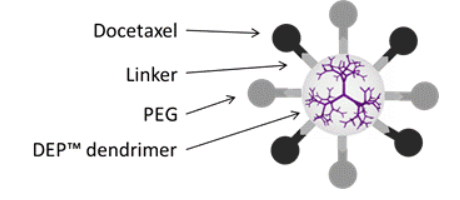
Each DEP® docetaxel molecule comprises docetaxel attached to a dendrimer scaffold. The linker between the docetaxel and the dendrimer is designed to release the docetaxel in a controlled manner.
Features & Benefits
- Therapeutic Potential and Benefits
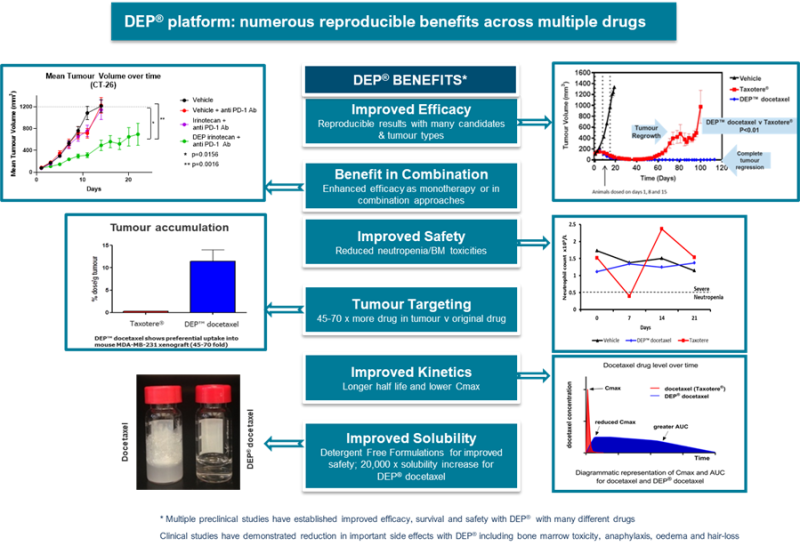
Superiority to docetaxel/Taxotere® Alone Across Multiple Cancer Types
In xenograft studies DEP® docetaxel demonstrated a significant enhancement of anticancer effect when compared to docetaxel/Taxotere® alone. Furthermore, in the study 60% of animals treated with DEP® docetaxel had no evidence of tumors at 94 days - whereas 100% of the docetaxel treated mice showed significant tumor re-growth or recurrence at the same time point.
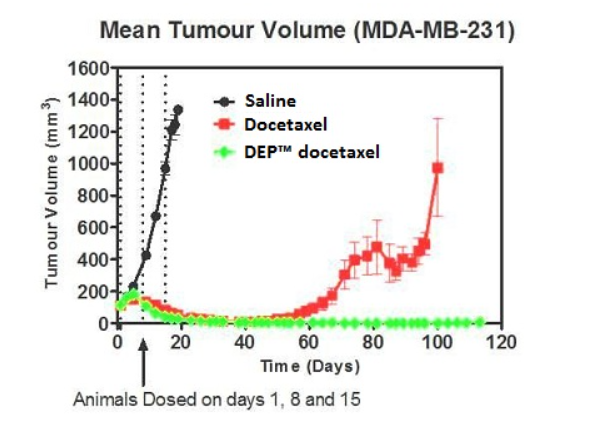
In hollow fiber studies DEP® docetaxel had significantly superior anti-cancer effects in vivo across a range of important cancer types when compared to Taxotere®. Breast, prostate, lung and ovarian tumor types were tested. In each case DEP® docetaxel was seen to significantly outperform the leading drug Taxotere®.
Preventing Neutropenia May Allow More Effective Treatment and Avoid the Need for Rescue Therapy and Hospitalization
Neutropenia is the major dose-limiting toxicity for marketed formulations of docetaxel. Currently, rescue therapy for neutropenia can require expensive treatment with the growth factor G-CSF. G-CSF therapy still leaves the possibility of severe illness, hospitalization or death from infection. In animal studies it has been found that DEP® docetaxel eliminates the neutropenia or thrombocytopenia caused by Taxotere® alone.
Elimination of Polysorbate 80 Reduces Risk of Anaphylactic Reactions & May Remove Need for Premedication with Corticosteroids
Currently pre-treatment with corticosteroids is required before using Taxotere® or any marketed docetaxel formulation due to the inclusion of the surfactant polysorbate 80, which can lead to anaphylactic shock. Even with prophylaxis potentially fatal anaphylaxis can still occur.
The absence of surfactant in the DEP® docetaxel formulation may avoid the need for such prophylaxis, and avoid the risk of polysorbate 80 related anaphylactic fatalities.
Tumor Targeting
DEP® docetaxel has been shown to preferentially accumulate in tumor tissues, with drug levels 40 times greater than are seen with conventional formulations of docetaxel. This is evidence of the significant tumor-targeting effect of Starpharma’s dendrimer formulation.
Extended Drug Half-Life
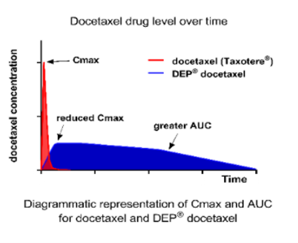
In pre-clinical models DEP® docetaxel has a plasma half-life of between 40 to 60 hours, more than 40 times longer than for docetaxel alone. It is believed this is a contributing factor to the improved efficacy of DEP® docetaxel.
- Advantages
The advantages* of DEP® docetaxel include:
- Reduction in neutropenia
- No steroid pre-treatment
- Tumor-targeting (~70x more), improved efficacy
- Improved pharmacokinetics
Clinical studies have demonstrated reduction in important side effects with DEP® including bone marrow toxicity, anaphylaxis, oedema, and hair-loss. DEP® docetaxel has patent filings to 2032 (plus up to an additional ~5 years).
*Multiple preclinical studies have established improved efficacy, survival, and safety with DEP® with many different drugs.
Applications & Uses
- Markets
- Uses
Docetaxel is a leading chemotherapy drug used to treat a wide range of solid tumors including breast, lung and prostate. It is marketed by Sanofi Aventis as Taxotere® and generated sales in excess of US$3 billion in 2010.
In pre-clinical studies DEP® docetaxel was shown to have substantially better efficacy and lower toxicity than Taxotere® alone. Of particular significance was the absence of neutropenia, the major dose-limiting toxicity for Taxotere®.
- Wider Applications
Wider Applications of the DEP® Dendrimer Drug Delivery Platform
Starpharma applies its DEP® dendrimer drug delivery platform to its own pipeline, and in collaboration with a number of well known pharmaceutical companies.
DEP™ is applicable to small molecules, peptides, proteins and antibodies, yielding benefits such as efficacy, targeting, improved pharmacokinetics and reduced toxicity, as well as greatly increased aqueous solubility.
Applying DEP® in this way has the potential to create innovative products with increased patient compliance and effectiveness and also provide companies with a unique market position leading to enhanced commercial returns.
DEP™ docetaxel is in Phase 2 clinical trials.
Regulatory & Compliance
- Certifications & Compliance
Technical Details & Test Data
- DEP® docetaxel clinical status
DEP® docetaxel phase 2 program is ongoing and includes both a monotherapy arm and the use of the product in combination with other anti-cancer treatment(s). The monotherapy trial is an open-label trial, with the objective of establishing anti-tumor activity (efficacy) & safety.
Interim Observations
- Efficacy signals observed, including prolonged stable disease and tumor shrinkage in patients with pancreatic, oesophageal, and gastric cancer. These tumor responses include stable disease for up to 40 weeks and significant tumor shrinkage in a heavily pre-treated oesophageal cancer patient, maintained for more than 28 weeks.
- Notable lack of bone marrow toxicity (e.g. neutropenia) and other common side effects including hair-loss, mouth ulcers, anaphylaxis and oedema
- Efficacy signals observed in heavily pre-treated patients (treated with up to 40 cycles and 9 different anti-cancer regimens previously)
Combination program includes DEP® docetaxel + nintedanib (Vargatef®) and DEP® docetaxel + gemcitabine (Gemzar®).
- DEP® docetaxel Phase 1 Results
- No steroid pre-treatment required due to DEP® docetaxel’s detergent-free formulation - unlike Taxotere®
- No neutropenia (compares to >>90% with Taxotere®)
- No protocol-defined Dose Limiting Toxicities
- Only one patient (1/27) with mild alopecia/hair loss – compared to ~75% with Taxotere®
- No reports of other problematic adverse events observed with docetaxel treatment, including anaphylaxis, fluid retention, diarrhea and nail disorders
- Encouraging efficacy signals in 13/27 DEP® docetaxel patients including:
- Stable disease (SD) in multiple patients with lung, pancreatic (SD>20 weeks), gastro-oesophageal (SD >18 weeks) cancers, and in other patients with glioblastoma (brain) and renal cancers.
- DEP® docetaxel Preclinical Results
Preclinical studies demonstrated that DEP® docetaxel achieved significantly superior anti-cancer effects across a range of important cancer types when compared to Taxotere® (standard docetaxel).