Knowde Enhanced TDS
Identification & Functionality
- Chemical Family
- Chemical Name
- Product Type
- Plastics & Elastomers Functions
- Technologies
- Product Families
- Chemical Structure
Features & Benefits
- Ready-to-Use Product Features
- Product Highlights
- Conventional fluids are the well-known general purpose silicones described in chemical notation as polydimethylsiloxanes. They are commercially produced in viscosities ranging from 0.65 to 2,500,000 cSt.
- Conventional silicone fluids are composed of polymer chains with unique flexibility. Polydimethylsiloxane has virtually no energy barrier for rotation. This results in one of the lowest glasstransition temperatures of any polymer.
- The liquid surface tension of polydimethylsiloxane is lower than the critical surface tension of wetting (24 dynes/cm). This causes polymers to spread over their own adsorbed films. An important consequence of the low intermolecular forces in polysiloxanes is the highest permeability coefficients of any polymer for oxygen and nitrogen.
- The fluids are thermally stable indefinately at 150°C in air. Fluids with viscosities of 50 cSt. or greater have negligible vapor pressure.
- At viscosities greater than 1,000 cSt. correlating to molecular weights greater than 30,000, polymer chain entanglement occurs, resulting in leveling of physical property change vs. viscosity. Refractive index, surface tension, density and viscosity-temperature coefficients are strikingly flat.
Applications & Uses
- Application Area
- Compatible Polymers & Resins
Properties
- Physical Form
- Mechanical Properties
- Typical Properties
- Thermal Properties
- Electrical Properties
- Optical Properties
- Solubility
- Acoustical Properties
Fluid Viscosity (cSt.)
Velocity of sound, m/s 30°C 50.7°C 0.65 873 795 2 931 863 20 975 918 100 985 930 1,000 987 933 - Polydimethylsiloxanes Properties
- Reactivity:
While they exhibit low reactivity under many conditions, certain environments are destructive to silicone fluids. Hydrogen fluoride, for example, attacks the silicon-oxygen bond to produce dimethylsilyl fluorides and water, which generate corrosive gasses. Strong bases such as methanolic potassium hydroxide destroy silicone fluids and create resinous byproducts. Thermal degradation at elevated temperatures causes rearrangement of the silicon-oxygen bonds to product volatile byproducts. Free-radical reaction of the methyl groups to form cross-linked materials by oxidation with peroxy compounds increases fluid viscosity and causes the fluid to gel.
- Solubility of Fluids:
Methylene chloride, chlorofluorocarbons, ethyl ether, xylene and methylethyl ketone are typical solvents for dimethylsiloxanes. Low viscosity polymers are also soluble in acetone, ethanol, dioxane and dihexyladipate. They are insoluble in methanol, cyclohexanol and ethylene glycol. The solubility parameter for 100 cSt. fluid is 7.4.
- Solubility of Water:
The equilibrium water absorption of silicones is 100-200ppm at 50-85% relative humidity. Drying of fluids is recommended for maximum performance in electrical applications. A typical drying protocol is to apply 1mm vacuum for 1 hour, which typically reduces water levels below 25ppm.
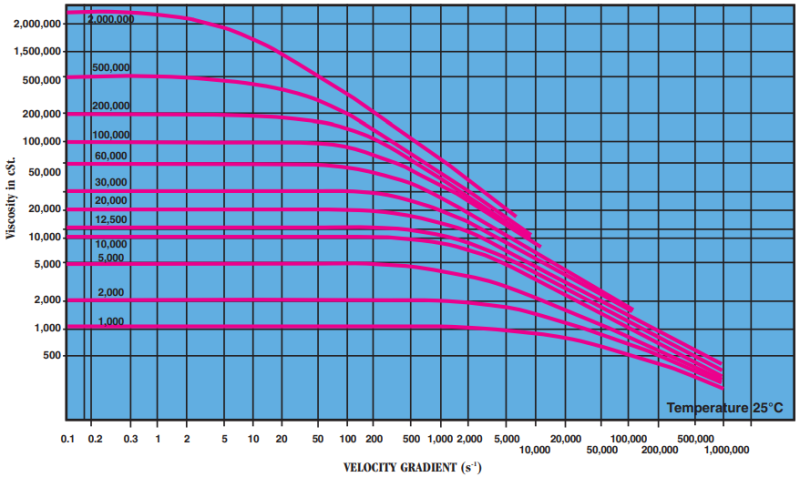
- Rheological Behavior Under Shear
At shear rates commonly encountered (≤10⁴ s⁻¹) polydimethylsiloxanes behave, at viscosities up to 1,000 cSt., like Newtonian fluids. Viscosity is constant and independent of the velocity gradient. Apparent viscosity is identical with viscosity extrapolated to zero velocity gradient. For oils of a higher viscosity than 1,000 cSt., this ratio is only constant for velocity gradients below a certain value. Beyond this value, becoming lower as the product becomes more viscous the ratio is no longer constant: apparent viscosity falls below real viscosity (extrapolated for a zero velocity gradient) and the behavior is then known as “pseudoplastic.” This change is perfectly reversible, and behavior again becomes Newtonian when the velocity gradient falls once more below the critical value. Viscosity returns to its initial level even after intense shearing of long duration. As a guide, the table indicates the “critical” velocity gradients for polydimethylsiloxanes (where change of rheological behavior occurs) as well as apparent viscosity measured at velocity gradient equal to 10,000 s⁻¹.
Critical velocity gradient (s⁻¹) Apparent viscosity for a velocity gradient of 10,000 s-1 (in cSt.)
1,000 2,500 850 12,500 200 4,700 30000 150 6,000 100,000 30 8,200 Apparent viscosity as a function of velocity gradient:
| Value | Units | Test Method / Conditions | |
| Coefficient of Adiabatic Compressibility | 1.10 x 10⁻¹⁰ | cm2 /dyne | — |
| Volume Reduction (of 100 cSt. fluid at 1,000 psi) | 0.70 - 0.75 | % | — |
| Volume Reduction (of 100 cSt. fluid at 10,000 psi) | 5.50 - 5.90 | % | — |
| Volume Reduction (of 100 cSt. fluid at 20,000 psi) | 9.00 - 9.20 | % | — |
| Volume Reduction (of 100 cSt. fluid at 40,000 psi) | 13.30 - 13.80 | % | — |
| Value | Units | Test Method / Conditions | |
| Density | 0.966 | g/ml | — |
| Dielectric Strength | 400.0 | V/m | — |
| Refractive Index (at 20°C) | 1.4025 | — | — |
| Viscosity (at 25°C) | 100.0 | cSt | — |
| Viscosity Temperature Coefficient | 0.6 | — | — |
| Pour Point | -65.0 | °C | — |
| Specific Gravity | 0.966 | — | — |
| Surface Tension | 20.9 | mN/m | — |
| Dielectric Constant | 2.75 | F/m | — |
| Flash Point | 315.0 | °C | — |
| Molecular Weight | 5970.0 | g/mol | — |
| Value | Units | Test Method / Conditions | |
| Coefficient of Thermal Expansion | 9.3 x 10⁻⁴ | °C⁻¹ | — |
| Thermal Conductivity | 3.7 x 10⁻⁴ | cal/cm. sec. °C | — |
| Specific Heat | 0.35 - 0.37 | cal/gm/°C | — |
| Heat of Formation | -2.41 | kcal/gm | — |
| Heat of Combustion (>50 cSt.) | 6.13 | kcal/gm | — |
| Glass Transistion Temperature (Tg) | -128.0 | °C | — |
| Gel Time (at 150°C) | Indefinite | — | — |
| Gel Time (for intermediate viscosity fluids at 200°C) | 200.0 | hours | — |
| Gel Time (for high viscosity fluids at 200°C) | 100.0 | hours | — |
| Auto-ignition Temperature (for fluids >10 cSt) | min. 460 | °C | — |
| Value | Units | Test Method / Conditions | |
| Dissipation Factor | 0.0001 | — | — |
| Volume Resistivity (at 20°C) | 1x10¹⁵ | ohm-cm | — |
| Value | Units | Test Method / Conditions | |
| Refractive Index (at 25°C) | 1.397 - 1.404 | — | — |
| Verdet Constant of Magnetic Rotary Power | 16.2 - 16.9 x 10⁻³ | mm/gm/cm | — |
| Value | Units | Test Method / Conditions | |
| Nitrogen (at 25°C) | 0.16 - 0.17 | ml gas/ml liquid | — |
| Carbon Dioxide (at 25°C) | 1.0 | ml gas/ml liquid | — |
| Air (at 25°C) | 0.16 - 0.19 | ml gas/ml liquid | — |
| Hydrogen (at 25°C) | 0.11 - 0.12 | ml gas/ml liquid | — |
Regulatory & Compliance
- Chemical Inventories
Technical Details & Test Data
- Test Data
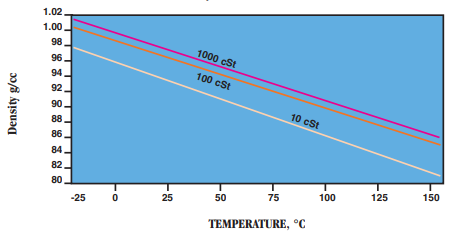
- Density:
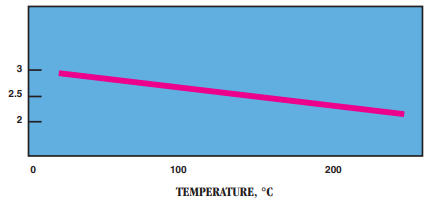
- Dielectric Constant:
- Dielectric Strength in kV/mm:
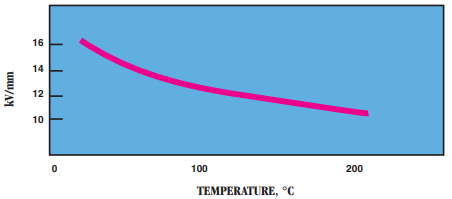
- Power Factor:
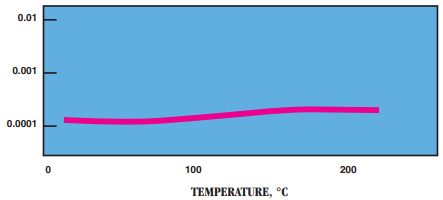
- Moisture Absorption vs. Resistivity:
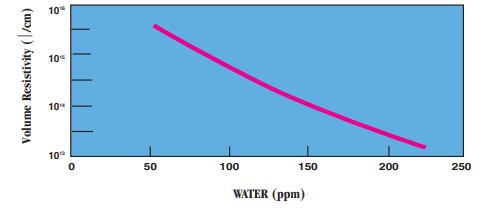
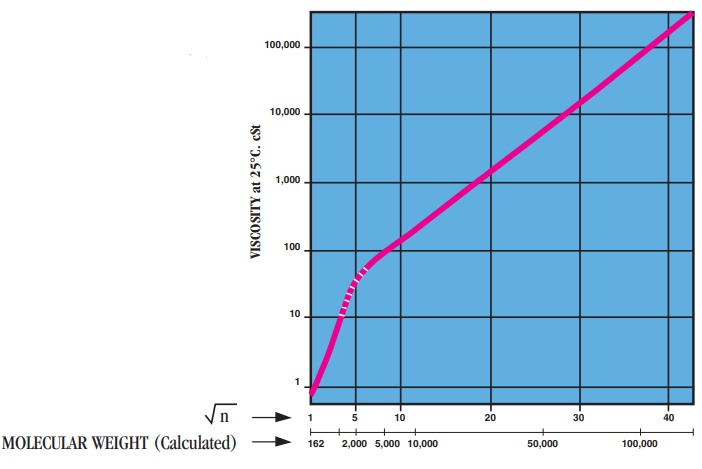
- Molecular Weight
Viscosity, μ, of Polydimethylsiloxanes as a function of a degree of polymerization “n”.
Note: The straight portion of the slope corresponds to A.J. Barry's relationship on molecular weights>2,500:
log μ꜀ₛₜ= 1.00 + 0.0123M⁰.⁵
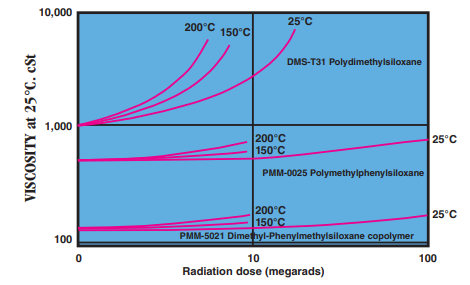
- Radiation Resistance
Effect of gamma radiation on viscosity of silicone fluids
Packaging & Availability
- Standard Packaging
- 100 g
- 3 kg
- 16 kg
- 190 kg