Knowde Enhanced TDS
Identification & Functionality
- Ingredient Name
- Food Ingredients Functions
- Pharma & Nutraceuticals Functions
Features & Benefits
- Benefit Claims (Health)
- Food Ingredients Features
- Product Highlights
- Clinically proven benefits to intestinal health and immunity in infants and young children and combined effect with drugs on rotaviral enteritis
- Clinically proven benefits to gut microbiota and bowel movement
- Specific probiotic strains naturally originate from humans and conventional food
- Strain patents in China, Taiwan and USA
- Strain deposit: BLI-02 at BCRC (910812); MP108 at BCRC (910483)
- Product Features
- Better efficacy
- Stronger immunity
- Shorter recovery time
- Function: Glucose Control
Applications & Uses
- Applications
- Food & Nutrition Applications
- Dosage Information
Daily dosage: 9 x 10° CFU for infants under 2 years and 1.8 x 101 CFU for children over 2 years and adults
- Product Applications
- Functional foods and dietary supplements in forms of capsules, tablets, powdered sachets, and others
- IP0301: help support gut health, relieve rotaviral enteritis and promotes intestinal function recovery after colorectal surgery
Properties
- Specifications
- Contents
- Bifidobacterium animalis subsp. lactis BB-115
- Bifidobacterium longum subsp. infantis BLI-02
- Lactobacillus rhamnosus MP108
| Value | Units | Test Method / Conditions | |
| Specification | 10¹¹ | cfu/g | — |
Regulatory & Compliance
- Certifications & Compliance
Technical Details & Test Data
- Clinical Study
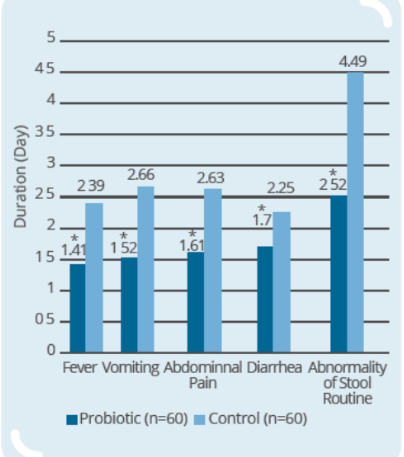
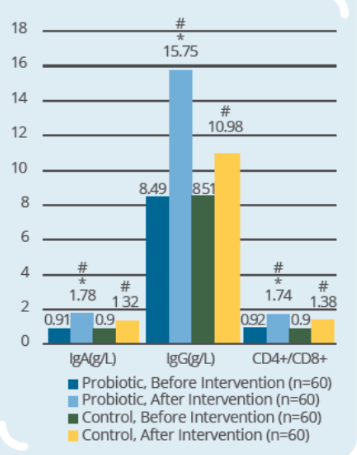
Clinical Study on 120 infants and young children (9-56 months old) with rotaviral enteritis for 3 days, with 60 subjects in control group (conventional drugs) and 60 subjects in probiotic group (conventional drugs + IP0301) (* p < 0.05 vs. control, # p < 0.05 vs. the same group before intervention)
- IP0301 has beneficial effect on rotaviral enteritis treatment
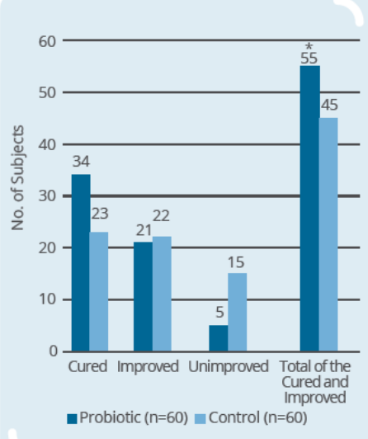
- IP0301 helps relieve symptoms of rotaviral enteritis
- IP0301 helps enhance immunity more
Clinical Study on 90 subjects undergoing colorectal resection, with 45 subjects in control group (surgery only) and 45 subjects in probiotic group (IP0301 from 8 days to 1 day prior to surgery) (* p ≤ 0.05 vs. control, # p≤ 0.05 vs. the same group before surgery)- IP0301 helps recover from colorectal surgery
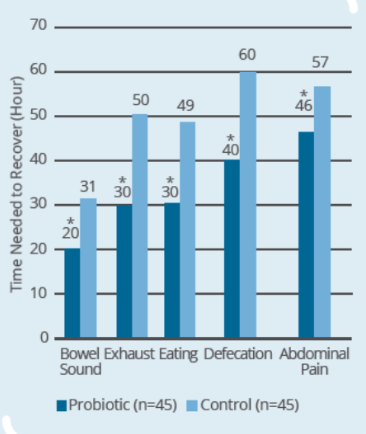
- IP0301 helps improve gut microbiota after colorectal surgery
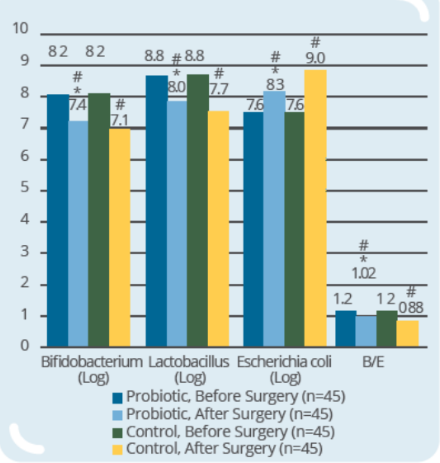
- IP0301 helps enhance immunity after colorectal surgery
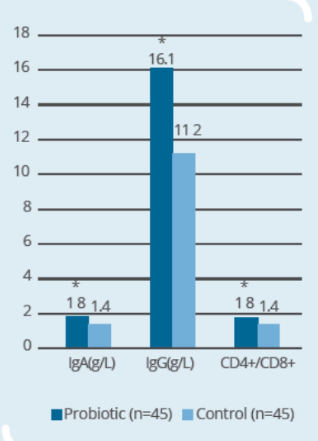
Storage & Handling
- Shelf Life
- 2 years
- Storage and Shelf Life Conditions
- Shelf life: 2 years
- Storage: -16 to -20°C
