Knowde Enhanced TDS
Identification & Functionality
- Chemical Family
- Chemical Name
Features & Benefits
- Labeling Claims
- Base Chemicals Features
- Doped Aluminas and Mixed Oxides Highlights
- Silica-aluminas exhibit a higher degree of acidity than pure aluminas. The hydrates are sold under the trade name SIRAL and the corresponding oxides as SIRALOX.
- Mg/Al mixed hydroxides and oxides exhibiting more basic surface sites are indispensable support materials for diverse applications. These materials are sold as PURAL MG and PURALOX MG.
- Besides the formation of mixed oxides, also the addition of other elements to the pure alumina, silica-alumina or mixed Al/Mg oxides and hydroxides is a facile way to further improve the properties of the corresponding starting materials for several applications. For example, lanthanum-doped aluminas exhibit a much higher thermostability in comparison to the non-doped counterparts.
Applications & Uses
- Markets
- Applications
- Applicable Processes
- Base Chemicals End Uses
Properties
- Physico-Chemical Properties
- Note
* After activation at 550°C for 3 hours
| Value | Units | Test Method / Conditions | |
| Al₂O₃ | 60.0 | % | — |
| SiO₂ | 40.0 | % | — |
| LOI | 20.0 | % | — |
| Loose Bulk Density | 100 - 300 | g/l | — |
| Particle Size (d50) | 35.0 | μm | — |
| Surface Area * | 500.0 | m²/g | Brunauer-Emmett-Teller Method |
| Pore Volume* | 1.5 | ml/g | — |
Technical Details & Test Data
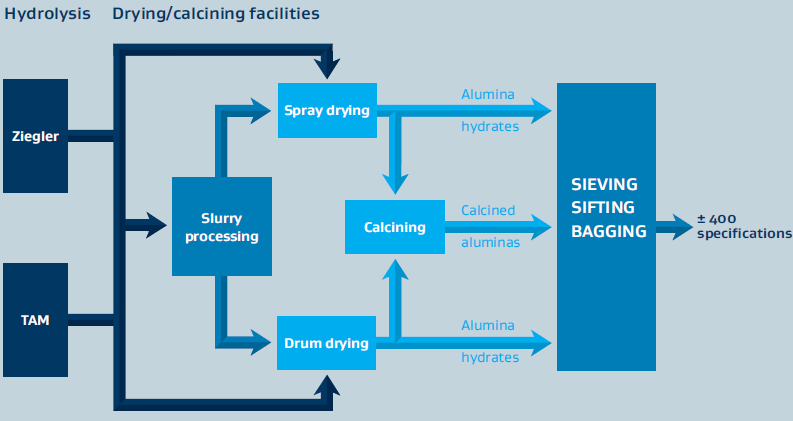
- Alumina Production Process
Sasol Inorganics produces high- and ultra-high-purity aluminas primarily through synthetic aluminum alkoxide processing routes. The alumina is produced either as a co-product with synthetic linear alcohols (Ziegler method) or directly from aluminum metal (on-purpose route). Several production steps must be completed to produce the different alumina-based products. In the first step, an aqueous intermediate (alumina slurry) is produced, which is further tailored in the subsequent processing steps to obtain the various products sold on the market. These can be alumina hydrates, calcined aluminas, and doped versions thereof.
Figure 1: Schematic for the Alumina Manufacturing Process
- Test Data of SIRAL Products
Silica-alumina hydrates (SIRAL) and the corresponding oxides (SIRALOX) exhibit characteristics which differ significantly from pure aluminas. One of the key properties of silica-aluminas is their comparatively high level of surface acidity, making them useful catalyst support materials, for example in the field of refinery catalyst or in diesel-engine applications.
The concentration and type of acidic sites (Lewis-acidic sites versus Brønsted-acidic sites) present in the materials strongly depends on Al2O3/SiO2 ratio as it can be seen from figure1. In general, the total acidity is governed by the Lewis-acidic sites. The highest concentration of Lewis-acidic sites is observed for SIRAL 5 while the highest amount of Brønsted-acidic sites is found in SIRAL 30 and SIRAL 40.
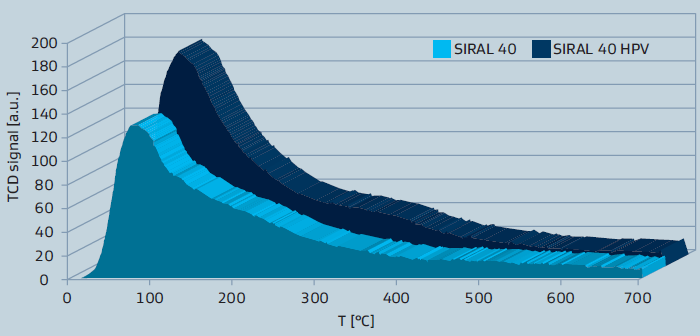
The more recently developed SIRAL HPV series provides even higher acidity and exhibits a larger pore volume. The higher acidity is shown by NH3-TPD curves in figure 2 for SIRAL 40.
Figure 1: Acidity of SIRAL after calcination at 900°C
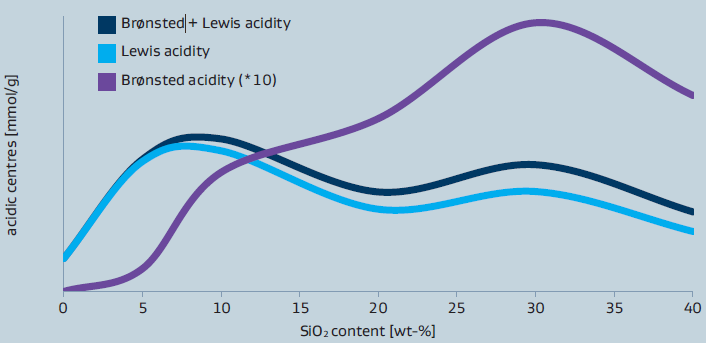
Figure 2: NH3-TPD of SIRAL 40 types after calcination at 900°C
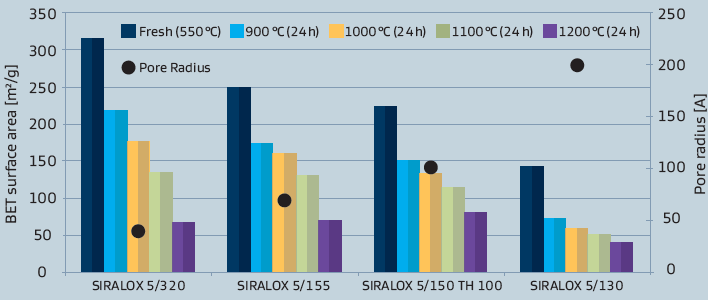
Besides differences in the chemical properties, also some physical characteristics like surface area are in marked contrast to pure aluminas as illustrated in figure 3.As different applications also demand for different physical properties, these can be tailored by varying the base alumina.
The flexibility which is possible in case of the silica-aluminas is represented in figure 4. The different physical properties of SIRALOX 5 depend on the corresponding starting alumina.
Figure 3: Surface area of SIRALOX Effect of silica content and temperature
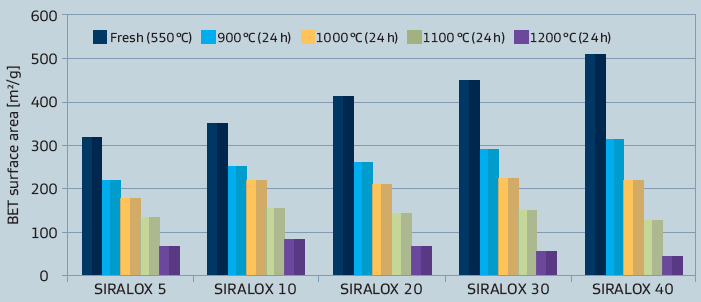
Figure 4: Characteristics of SIRALOX Effect of different starting aluminas
- Analytical Methods
- Element Analysis: Alumina powder is quantitatively brought into solution by using acids and then analyzed by ICP, atomic emission. Additionally, X-ray fluorescence spectroscopy is used.
- Crystallite Type and Average Crystalline Phases: Powdered samples of the alumina are analyzed by using X-Ray Diffractometry (XRD) on either a Siemens D5000 or a Philips X’Pert diffractometer. The resulting diffractogram enables the laboratory to identify the crystal structure of the material.
- Particle Size Distribution: The particle size distribution of alumina may be measured by various instruments, namely, Cilas Granulometer 1064 supplied by Quantachrome, Malvern Mastersizer or Luftstrahlsieb (air sieve) supplied by Alpine.
- Surface Area Analysis: The surface area of the alumina is measured by using an instrument supplied by Quantachrome (Nova series) or by Micromeritics (Gemini series). The method entails low temperature adsorption of nitrogen at the BET region of the adsorption isotherm.
- Pore Volume and Pore Size Distribution: The boehmite is first calcined at 550 °C for three hours in preparation for analysis. The porosity is measured by nitrogen desorption using Autosorb instruments supplied by Quantachrome.
- Differential Scanning Calorimetry (DSC): Netzsch STA 449C Jupiter, Setaram 92 or Perkin Elmer instruments may be used with a selected heating rate to obtain the exothermic and endothermic transitions of alumina. Additional test methods are available for other physical properties upon request.

