Knowde Enhanced TDS
Identification & Functionality
- Country of Origin
- Ingredient Name
- Food Ingredients Functions
- Ingredients
- Vinegar, Cultured Dextrose
- Technologies
- Product Families
- Composition
Vinegar, Cultured Dextrose
Features & Benefits
- Labeling Claims
- Food Ingredients Features
Applications & Uses
- Markets
- Food & Nutrition Applications
- Use Level
- 0.75 - 1.75%.
- Application Areas
Ready-to-Eat Meats, Brines and Marinades, Dressings, Sauces, Refrigerated Meals, RTE Side Dishes and Dips.
- Application Rates
Typical application rates vary based on the food formulation, manufacturing practices and other characteristics. Standard usage rates range from 0.75-1.75%.
- Best Practices
- Adherence to good manufacturing practices (GMPs) are required for optimal performance.
- Product should be added to an aqueous solution and mixed thoroughly to incorporate prior to the addition into a food formulation. This could include water, vinegar, etc.
- Final pH should be monitored after addition and adjusted, if needed.
Properties
- Physical Form
- Solubility
- Appearance
- Light tan free flowing powder
- Microbiological Values
| Value | Units | Test Method / Conditions | |
| Coliforms | max. 10 | CFU/g | — |
| E.coli | max. 3 | CFU/g | — |
| Listeria spp | Absent | CFU/25g | — |
| Salmonella | Absent | CFU/25g | — |
| Standard Plate Count | max. 50,000 | CFU/g | — |
| Yeast/Mold | max. 100 | CFU/g | — |
Regulatory & Compliance
- Certifications & Compliance
- Non-GMO Status
proVONTAGE® 463 is produced through a natural fermentation process by microorganisms that are not genetically modified. In addition, care is taken to only use ingredients that are also free from genetically modified organisms. As such regulation (EC) No. 1829/2003 is not applicable nor is there any labeling required as outlined in regulation (EC) No. 1829/2003 and 1830/2003.
Technical Details & Test Data
- Test Results
Lactic Acid Bacteria
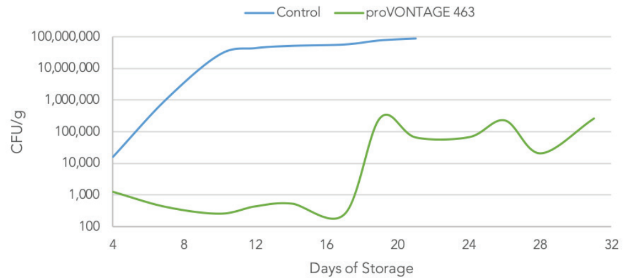
Lactic Acid Bacterial counts on MRS for marinated chicken breast stored at 4°C.
Aerobic Plate Count
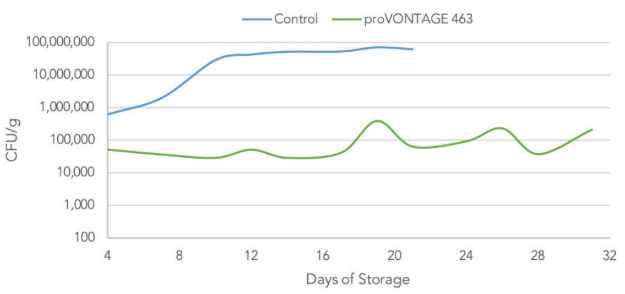
Aerobic Plate Count on TSB for marinated chicken breast stored at 4°C
Packaging & Availability
- Packaging Type
- Packaging Information
proVONTAGE® 463 is offered in 50lb (22.68 kg) paper bags with a food grade foil liner.
Storage & Handling
- Shelf Life
- 24 Months
- Storage Information
Product is best stored in unopened bags, in a cool (<80°F), and dry (RH <60%) environment.

